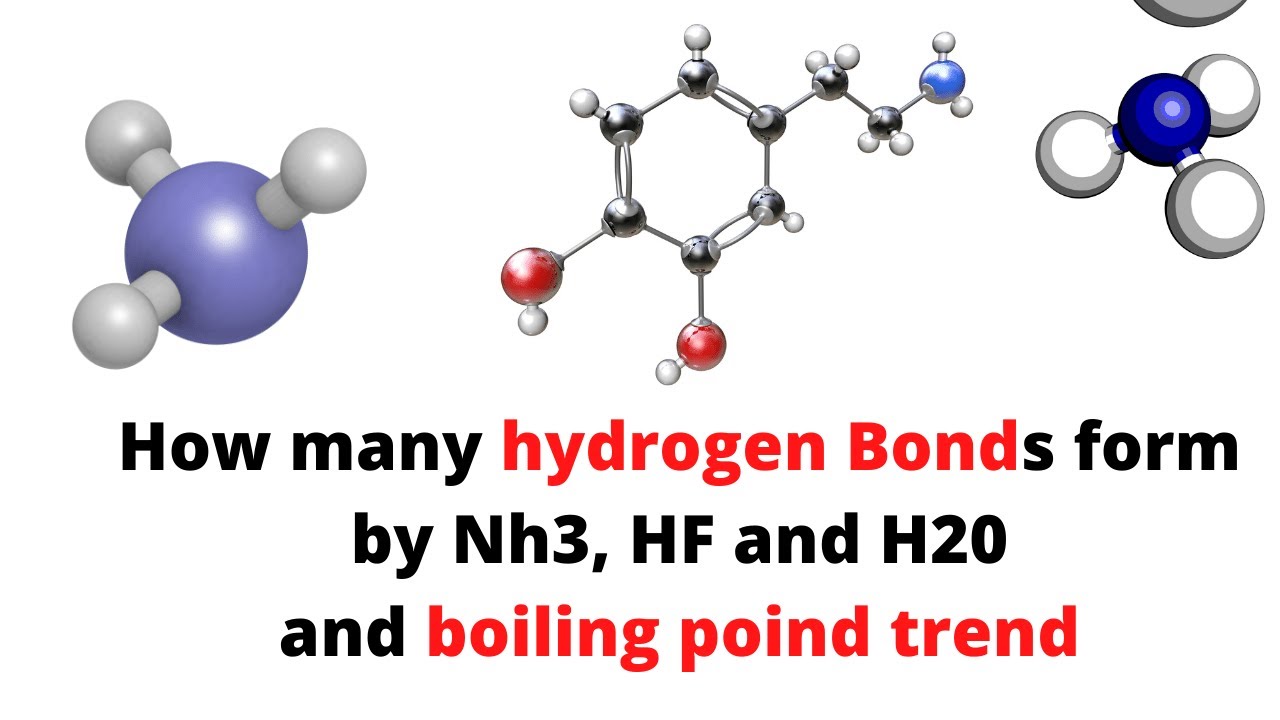
Can Nh3 Form Hydrogen Bonds
Can Nh3 Form Hydrogen Bonds - The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Ammonia molecules joined together by hydrogen bonds makeup ammonia. Yes, ammonia (nh3) can form hydrogen bonds. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Yes, ammonia (nh3) can form hydrogen bonds. However, forming hydrogen bonds is an. A molecule of ammonia can give and receive up to three hydrogen bonds. Nh3 (ammonia) does have hydrogen bonding.
This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. Yes, ammonia (nh3) can form hydrogen bonds. Yes, ammonia (nh3) can form hydrogen bonds. However, forming hydrogen bonds is an. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. A molecule of ammonia can give and receive up to three hydrogen bonds. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Nh3 (ammonia) does have hydrogen bonding. Ammonia molecules joined together by hydrogen bonds makeup ammonia.
Yes, ammonia (nh3) can form hydrogen bonds. Ammonia molecules joined together by hydrogen bonds makeup ammonia. Yes, ammonia (nh3) can form hydrogen bonds. However, forming hydrogen bonds is an. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Nh3 (ammonia) does have hydrogen bonding. A molecule of ammonia can give and receive up to three hydrogen bonds. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with.
Why can’t NH3 have 4 Hydrogen bonds like H2O? r/chemhelp
This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Yes, ammonia (nh3) can form hydrogen bonds. However, forming hydrogen bonds is an. Actually, nh3 is able to form hydrogen bonds with water.
Nh3 H2o Estudiar
A molecule of ammonia can give and receive up to three hydrogen bonds. Ammonia molecules joined together by hydrogen bonds makeup ammonia. However, forming hydrogen bonds is an. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Yes, ammonia (nh3) can form hydrogen bonds.
[Solved] Ammonia, NH3, exhibits hydrogen bonding. Using five molecules
Yes, ammonia (nh3) can form hydrogen bonds. A molecule of ammonia can give and receive up to three hydrogen bonds. Nh3 (ammonia) does have hydrogen bonding. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. However, forming hydrogen bonds is an.
hydrogen bond. chemistry lesson. Infographic. hydrogen bonds between
Ammonia molecules joined together by hydrogen bonds makeup ammonia. Actually, nh3 is able to form hydrogen bonds with water and even with itself. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Yes, ammonia (nh3) can form hydrogen bonds. This is because it contains a nitrogen atom (n), which is one.
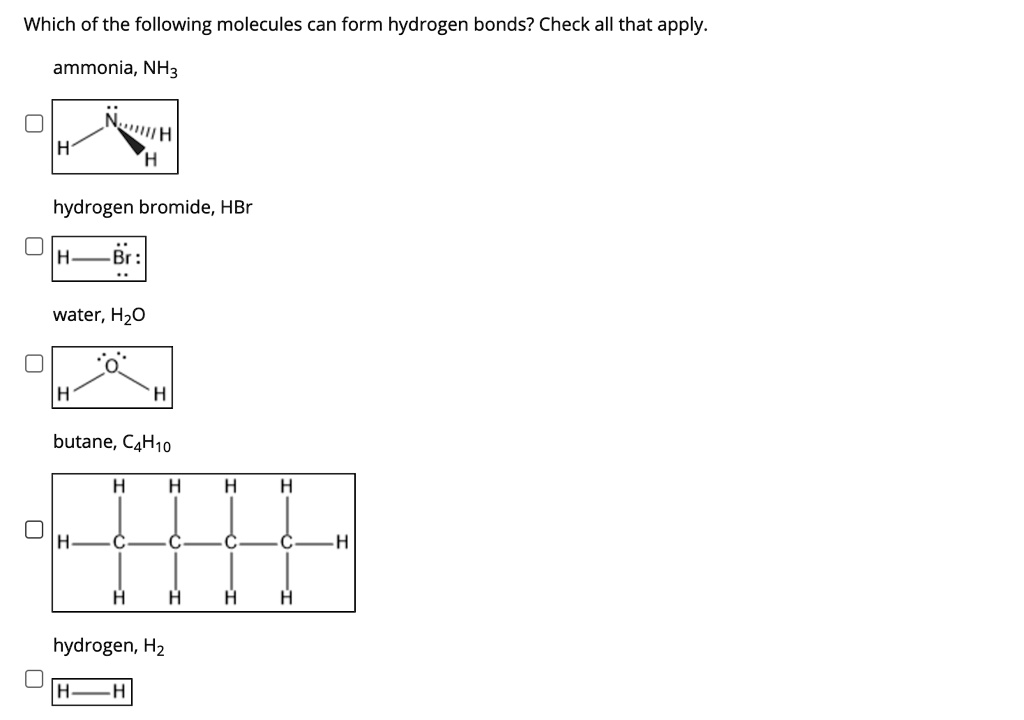
SOLVED Which of the following molecules can form hydrogen bonds? Check
Actually, nh3 is able to form hydrogen bonds with water and even with itself. A molecule of ammonia can give and receive up to three hydrogen bonds. Yes, ammonia (nh3) can form hydrogen bonds. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. However, forming hydrogen bonds is an.
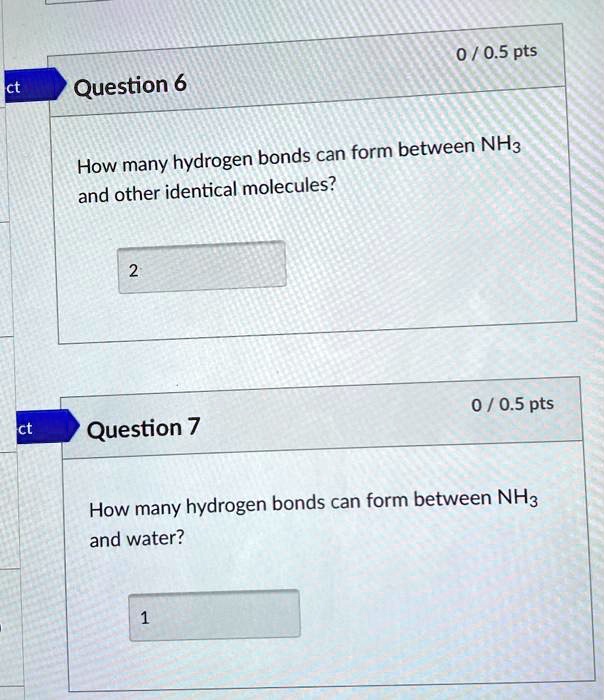
SOLVED 0 / 0.5 pts Question 6 How many hydrogen bonds can form between
This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. Yes, ammonia (nh3) can form hydrogen bonds. Nh3 (ammonia) does have hydrogen bonding. Yes, ammonia (nh3) can form hydrogen bonds. Actually, nh3 is able to form hydrogen bonds with water and even with itself.
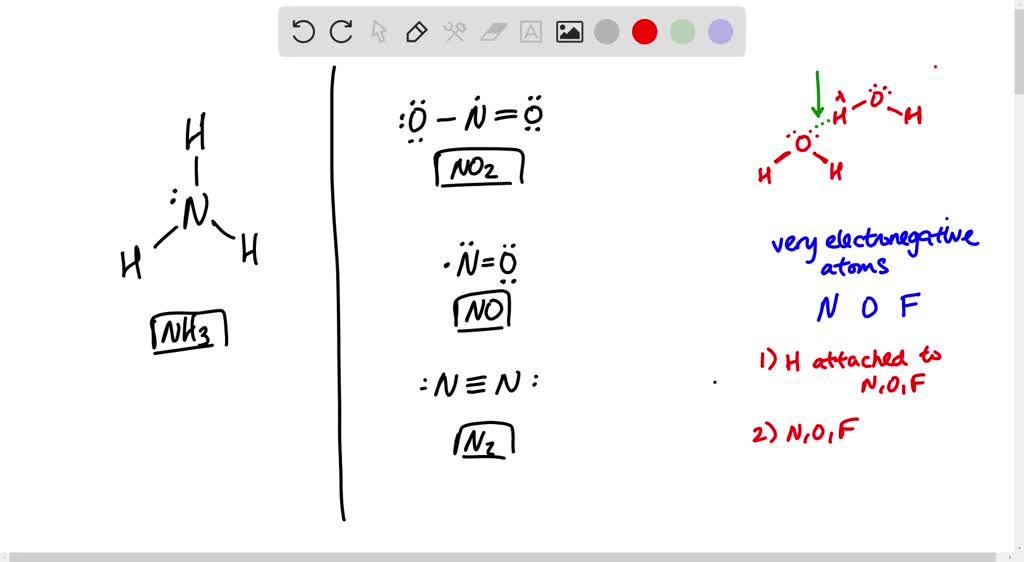
SOLVED Can NH3 form hydrogen bonds with NO2 or NO or N2?
Ammonia molecules joined together by hydrogen bonds makeup ammonia. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Yes, ammonia (nh3) can form hydrogen bonds. Actually, nh3 is able to form hydrogen bonds.
Types of Chemical Bonds
Nh3 (ammonia) does have hydrogen bonding. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Ammonia molecules joined together by hydrogen bonds makeup ammonia. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. However, forming hydrogen bonds is an.
SOLVED Which of these molecules can form hydrogen bonds between them
Actually, nh3 is able to form hydrogen bonds with water and even with itself. Ammonia molecules joined together by hydrogen bonds makeup ammonia. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being..
Hydrogen Bonding
Yes, ammonia (nh3) can form hydrogen bonds. Nh3 (ammonia) does have hydrogen bonding. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Ammonia molecules joined together by hydrogen bonds makeup ammonia. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being.
The Nitrogen Atom In Ammonia Has A Lone Pair Of Electrons, Which Can Form A Hydrogen Bond With.
A molecule of ammonia can give and receive up to three hydrogen bonds. Ammonia molecules joined together by hydrogen bonds makeup ammonia. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Yes, ammonia (nh3) can form hydrogen bonds.
This Is Because It Contains A Nitrogen Atom (N), Which Is One Of The Three Atoms (The Others Being.
Nh3 (ammonia) does have hydrogen bonding. However, forming hydrogen bonds is an. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Yes, ammonia (nh3) can form hydrogen bonds.