Lewis Dot Structure Practice Worksheet
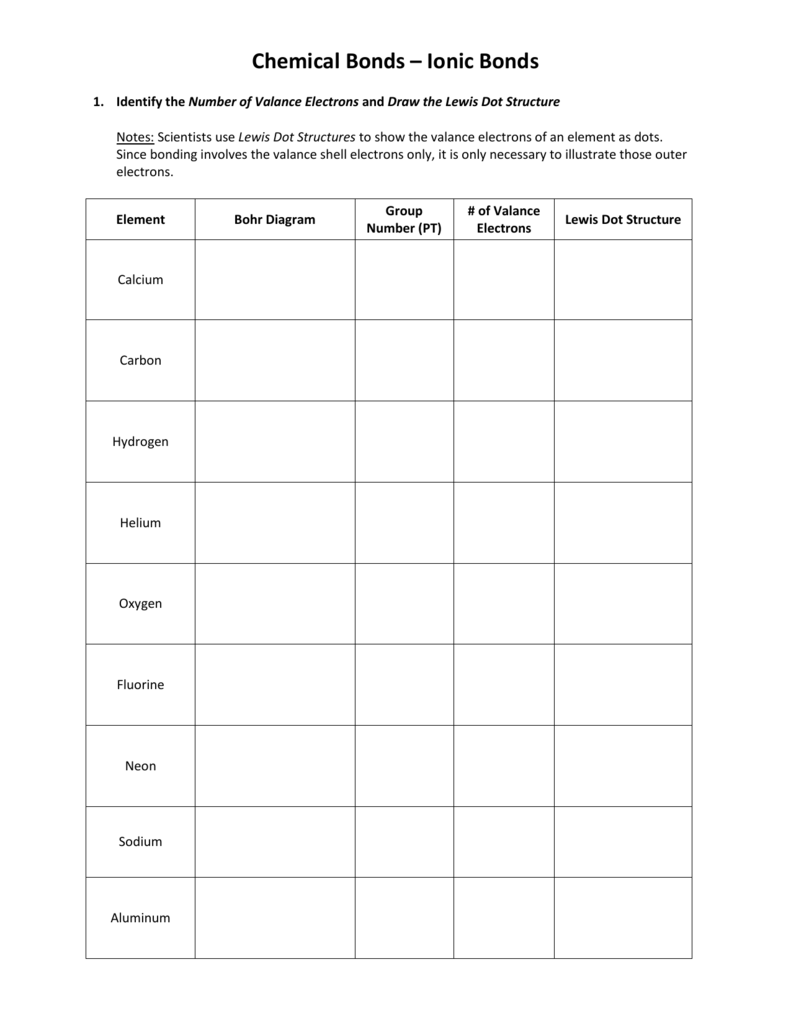
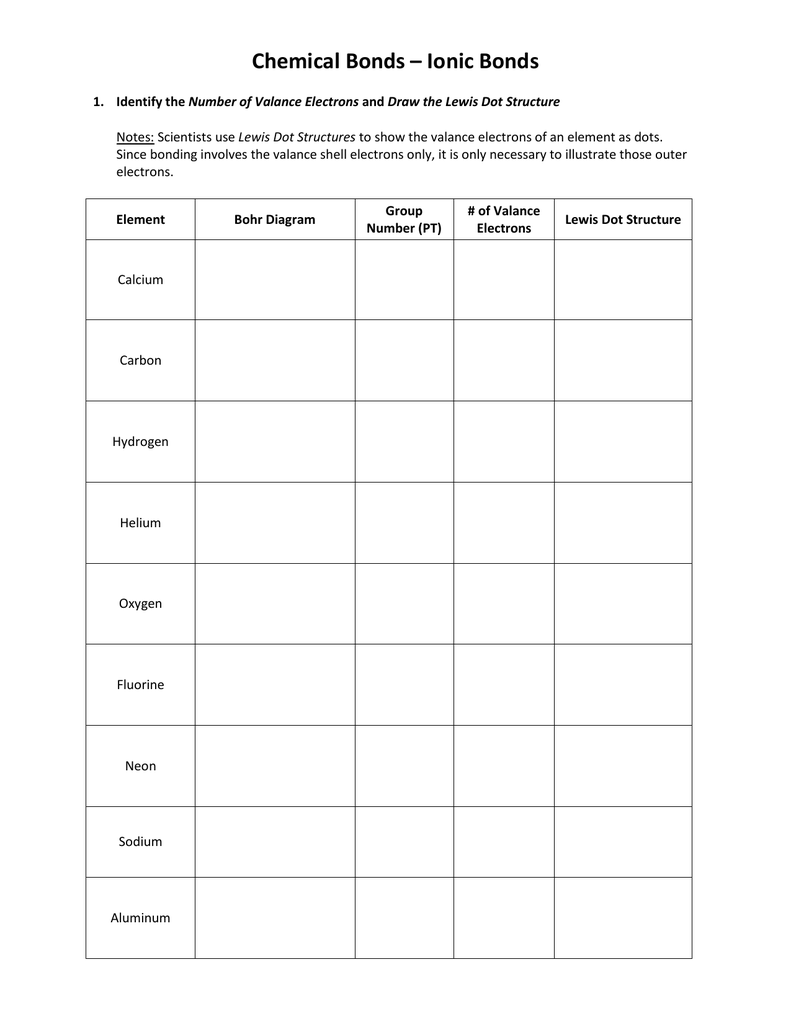
Lewis Dot Structure Practice Worksheet - For each of the following, draw the lewis dot structure, give the electron arrangement (e.a.) and the molecular geometry (m.g.): In the first box below the formula, write your first impression of the lewis formula. How could you use this pattern. Answer the following questions and check your answers below. Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule. Describe the pattern of the lewis dot structures of the first 18 elements (include periods and groups/families)? Check your answers with the key and explanations provided. Then, identify the most stable structure. Practice sheet electron dot (lewis) structures a lewis or electron dot structure is a convenient representation of the valence electrons in an. Identify the number of valance electrons and draw the lewis dot structure.
How could you use this pattern. In the second box, calculate s/2/b. In the first box below the formula, write your first impression of the lewis formula. Draw the lewis structures for 12 molecules and ions using the rules and a periodic table. Scientists use lewis dot structures to show the valance electrons of. For each of the following, draw the lewis dot structure, give the electron arrangement (e.a.) and the molecular geometry (m.g.): Answer the following questions and check your answers below. Identify the number of valance electrons and draw the lewis dot structure. Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule. Describe the pattern of the lewis dot structures of the first 18 elements (include periods and groups/families)?
Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule. In the first box below the formula, write your first impression of the lewis formula. For each of the following, draw the lewis dot structure, give the electron arrangement (e.a.) and the molecular geometry (m.g.): Answer the following questions and check your answers below. Draw the lewis structures for 12 molecules and ions using the rules and a periodic table. Identify the number of valance electrons and draw the lewis dot structure. Then, identify the most stable structure. Be sure you know how to draw. Describe the pattern of the lewis dot structures of the first 18 elements (include periods and groups/families)? Scientists use lewis dot structures to show the valance electrons of.
Chemistry Worksheet Lewis Dot Structures
Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule. Scientists use lewis dot structures to show the valance electrons of. In the first box below the formula, write your first impression of the lewis formula. Answer the following questions and check your answers below. For each of the following, draw the lewis.
Lewis Dot Structure Practice Worksheet —
Draw the lewis structures for 12 molecules and ions using the rules and a periodic table. Describe the pattern of the lewis dot structures of the first 18 elements (include periods and groups/families)? Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule. Identify the number of valance electrons and draw the lewis.
Lewis Dot Diagram Worksheet Answers Key Printable Word Searches
Answer the following questions and check your answers below. Then, identify the most stable structure. Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule. Scientists use lewis dot structures to show the valance electrons of. How could you use this pattern.
Lewis Structure Practice Worksheet
Be sure you know how to draw. Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule. Describe the pattern of the lewis dot structures of the first 18 elements (include periods and groups/families)? Practice sheet electron dot (lewis) structures a lewis or electron dot structure is a convenient representation of the valence.
Lewis Structure Practice Worksheet Lewis Dot Structure Worksheet
Describe the pattern of the lewis dot structures of the first 18 elements (include periods and groups/families)? Identify the number of valance electrons and draw the lewis dot structure. Scientists use lewis dot structures to show the valance electrons of. Practice sheet electron dot (lewis) structures a lewis or electron dot structure is a convenient representation of the valence electrons.
[DIAGRAM] Lewis Dot Diagrams Chemistry Handout Answers
These problems are for practice only will not be graded. Scientists use lewis dot structures to show the valance electrons of. Draw the lewis structures for 12 molecules and ions using the rules and a periodic table. Then, identify the most stable structure. Be sure you know how to draw.
Lewis Structure Drawing Practice
Scientists use lewis dot structures to show the valance electrons of. Identify the number of valance electrons and draw the lewis dot structure. Answer the following questions and check your answers below. Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule. Draw the lewis structures for 12 molecules and ions using the.
Lewis Dot Structures Worksheet
Draw the lewis structures for 12 molecules and ions using the rules and a periodic table. Answer the following questions and check your answers below. Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule. Describe the pattern of the lewis dot structures of the first 18 elements (include periods and groups/families)? Be.
Drawing Lewis Dot Structures Worksheet
Then, identify the most stable structure. These problems are for practice only will not be graded. Identify the number of valance electrons and draw the lewis dot structure. In the second box, calculate s/2/b. Draw the lewis structures for 12 molecules and ions using the rules and a periodic table.
Lewis Dot Structures Worksheet
Check your answers with the key and explanations provided. In the first box below the formula, write your first impression of the lewis formula. Identify the number of valance electrons and draw the lewis dot structure. In the second box, calculate s/2/b. For each of the following, draw the lewis dot structure, give the electron arrangement (e.a.) and the molecular.
Be Sure You Know How To Draw.
Describe the pattern of the lewis dot structures of the first 18 elements (include periods and groups/families)? In the first box below the formula, write your first impression of the lewis formula. Practice sheet electron dot (lewis) structures a lewis or electron dot structure is a convenient representation of the valence electrons in an. Then, identify the most stable structure.
In The Second Box, Calculate S/2/B.
These problems are for practice only will not be graded. Check your answers with the key and explanations provided. Draw the lewis structures for 12 molecules and ions using the rules and a periodic table. Scientists use lewis dot structures to show the valance electrons of.
How Could You Use This Pattern.
Answer the following questions and check your answers below. For each of the following, draw the lewis dot structure, give the electron arrangement (e.a.) and the molecular geometry (m.g.): Identify the number of valance electrons and draw the lewis dot structure. Draw three possible lewis structures for n2o and assign formal charges to the atoms in each molecule.





![[DIAGRAM] Lewis Dot Diagrams Chemistry Handout Answers](https://classworkassignmentanswers.weebly.com/uploads/7/9/5/5/79550400/lewis-dot-structures-4_orig.jpg)