Metals Usually Form What Type Of Ions
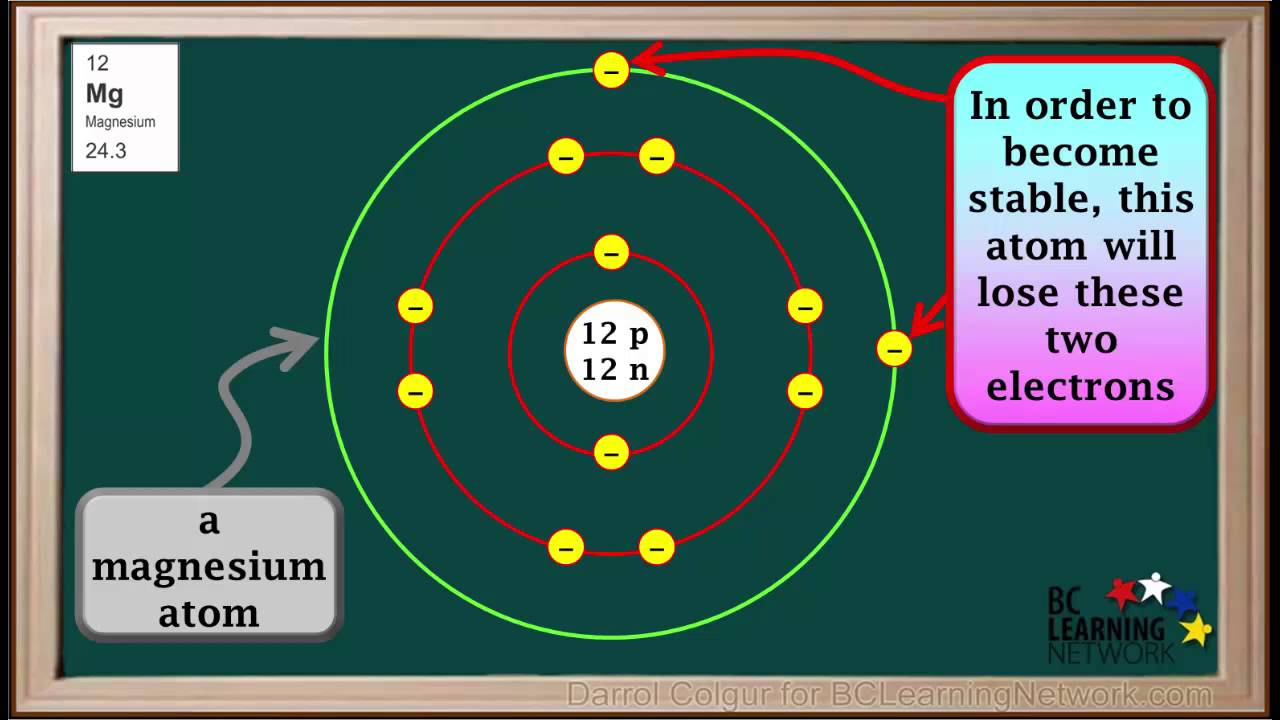
Metals Usually Form What Type Of Ions - Metals form positive ions, or cations. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the. Atoms achieve this type of configuration by gaining or losing electrons depending on the.
Atoms achieve this type of configuration by gaining or losing electrons depending on the. Metals form positive ions, or cations. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the.
Atoms achieve this type of configuration by gaining or losing electrons depending on the. Metals form positive ions, or cations. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the.
Periodic Table With Charges Of Ions Elcho Table
Atoms achieve this type of configuration by gaining or losing electrons depending on the. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Metals form positive ions, or cations. On the other side of the.
Do Metals Form Positive Or Negative Ions
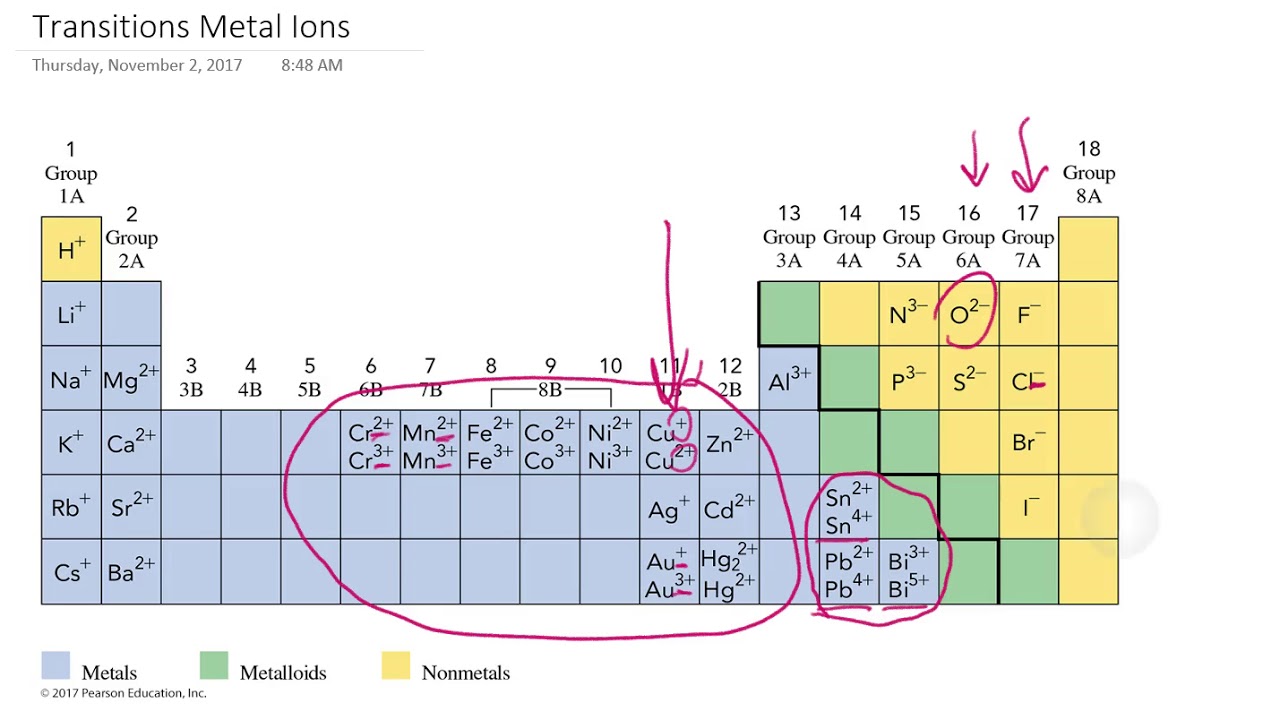
Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Metals form positive ions, or cations. Atoms achieve this type of configuration by gaining or losing electrons depending on the. On the other side of the.
Do Metals Form Positive Ions
Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Atoms achieve this type of configuration by gaining or losing electrons depending on the. Metals form positive ions, or cations. On the other side of the.
Chem Ions Scientific Tutor
Atoms achieve this type of configuration by gaining or losing electrons depending on the. On the other side of the. Metals form positive ions, or cations. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge.
metals tend to form what kind of ions Lombardi Bothe1936
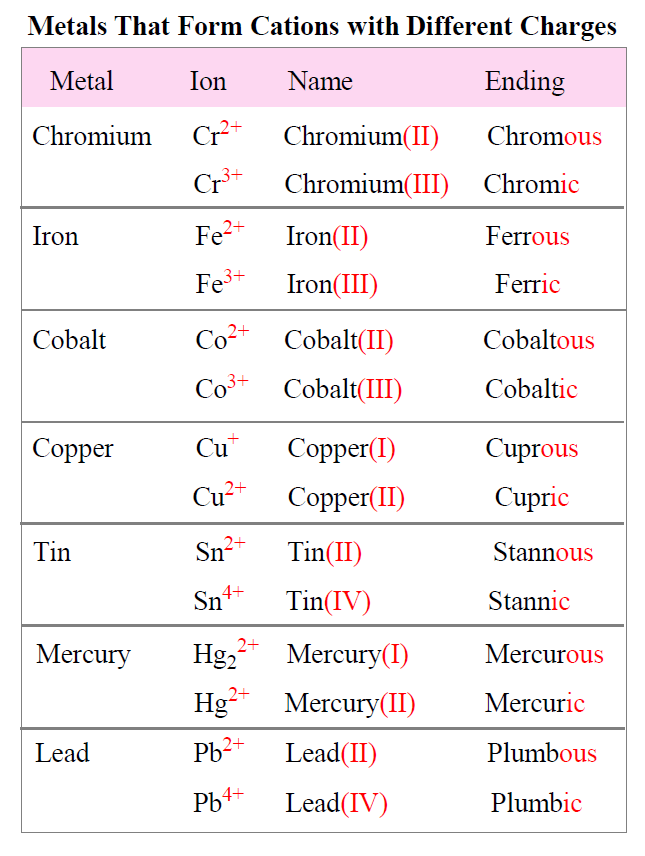
Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Metals form positive ions, or cations. Atoms achieve this type of configuration by gaining or losing electrons depending on the. On the other side of the.
Naming Monatomic and Polyatomic Ions Chemistry Steps
On the other side of the. Atoms achieve this type of configuration by gaining or losing electrons depending on the. Metals form positive ions, or cations. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge.
Do Metals Form Positive Or Negative Ions
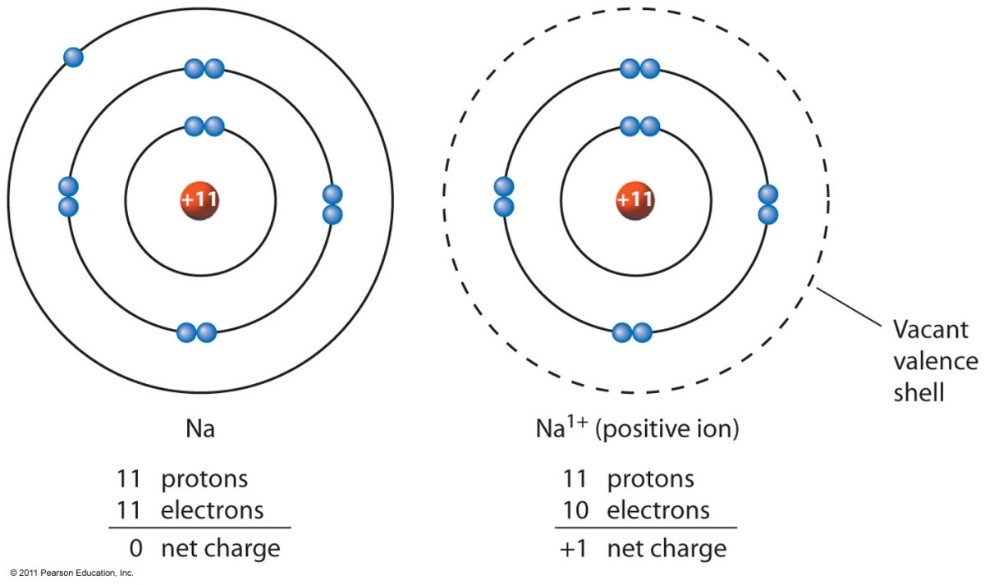
On the other side of the. Metals form positive ions, or cations. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Atoms achieve this type of configuration by gaining or losing electrons depending on the.
Do Metals Form Positive Or Negative Ions
Atoms achieve this type of configuration by gaining or losing electrons depending on the. On the other side of the. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Metals form positive ions, or cations.
PPT How do atoms form ions? PowerPoint Presentation, free download
Atoms achieve this type of configuration by gaining or losing electrons depending on the. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the. Metals form positive ions, or cations.
On The Other Side Of The.
Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Atoms achieve this type of configuration by gaining or losing electrons depending on the. Metals form positive ions, or cations.