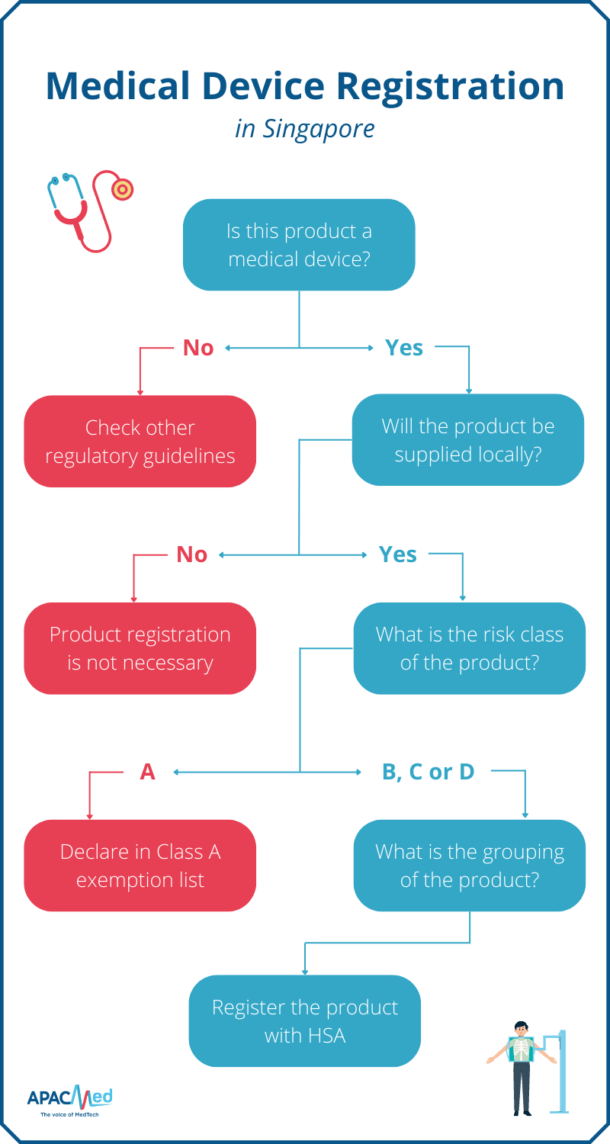
Regulations For Medical Devices
Regulations For Medical Devices - • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. (2) medical device classification and regulatory controls,. These final regulations codified in the cfr. Medical device problem reporting and the health care professional (pamphlet). This report describes (1) fda’s authority to regulate medical devices; Exemptions from federal preemption of state and local medical device requirements: Premarket notifications (510(k)), establishment registration,. Overview of regulations for medical devices:
This report describes (1) fda’s authority to regulate medical devices; These final regulations codified in the cfr. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. Exemptions from federal preemption of state and local medical device requirements: Overview of regulations for medical devices: (2) medical device classification and regulatory controls,. Premarket notifications (510(k)), establishment registration,. Medical device problem reporting and the health care professional (pamphlet).
Overview of regulations for medical devices: Medical device problem reporting and the health care professional (pamphlet). Premarket notifications (510(k)), establishment registration,. (2) medical device classification and regulatory controls,. Exemptions from federal preemption of state and local medical device requirements: • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. These final regulations codified in the cfr. This report describes (1) fda’s authority to regulate medical devices;
Shipping Regulations for Medical Devices Mercury Business Services
Medical device problem reporting and the health care professional (pamphlet). (2) medical device classification and regulatory controls,. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. Exemptions from federal preemption of state and local medical device requirements: Overview of regulations for medical devices:
FAQ on the European Medical Device Regulation B Medical Systems (US)
This report describes (1) fda’s authority to regulate medical devices; Premarket notifications (510(k)), establishment registration,. These final regulations codified in the cfr. Exemptions from federal preemption of state and local medical device requirements: Medical device problem reporting and the health care professional (pamphlet).
Medical Device Regulation Importance and Examples in APAC
Premarket notifications (510(k)), establishment registration,. Medical device problem reporting and the health care professional (pamphlet). (2) medical device classification and regulatory controls,. Exemptions from federal preemption of state and local medical device requirements: These final regulations codified in the cfr.
Medical Device Regulations
(2) medical device classification and regulatory controls,. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. Overview of regulations for medical devices: This report describes (1) fda’s authority to regulate medical devices; Premarket notifications (510(k)), establishment registration,.
Regulations of medical devices in india
These final regulations codified in the cfr. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. (2) medical device classification and regulatory controls,. Premarket notifications (510(k)), establishment registration,. Overview of regulations for medical devices:
The Interactive Guide Under The New EU Regulations on Medical Devices
• explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. Medical device problem reporting and the health care professional (pamphlet). These final regulations codified in the cfr. (2) medical device classification and regulatory controls,. Overview of regulations for medical devices:
Understanding Medical Devices Regulations to Guarantee Compliance
Medical device problem reporting and the health care professional (pamphlet). Premarket notifications (510(k)), establishment registration,. This report describes (1) fda’s authority to regulate medical devices; (2) medical device classification and regulatory controls,. Overview of regulations for medical devices:
Medical device regulations, classification & submissions Canada, US, EU
Medical device problem reporting and the health care professional (pamphlet). Exemptions from federal preemption of state and local medical device requirements: These final regulations codified in the cfr. (2) medical device classification and regulatory controls,. This report describes (1) fda’s authority to regulate medical devices;
FDA vs. EU Medical Device Regulation RAM Technologies
Exemptions from federal preemption of state and local medical device requirements: These final regulations codified in the cfr. Overview of regulations for medical devices: • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. Premarket notifications (510(k)), establishment registration,.
FDA Medical Device Regulation Guidance for 2022
Overview of regulations for medical devices: Exemptions from federal preemption of state and local medical device requirements: Medical device problem reporting and the health care professional (pamphlet). • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. This report describes (1) fda’s authority to.
Overview Of Regulations For Medical Devices:
• explain fda’s role in regulating medical devices • define a medical device and review basics about device classification • describe five steps to get a. This report describes (1) fda’s authority to regulate medical devices; (2) medical device classification and regulatory controls,. Exemptions from federal preemption of state and local medical device requirements:
Medical Device Problem Reporting And The Health Care Professional (Pamphlet).
These final regulations codified in the cfr. Premarket notifications (510(k)), establishment registration,.