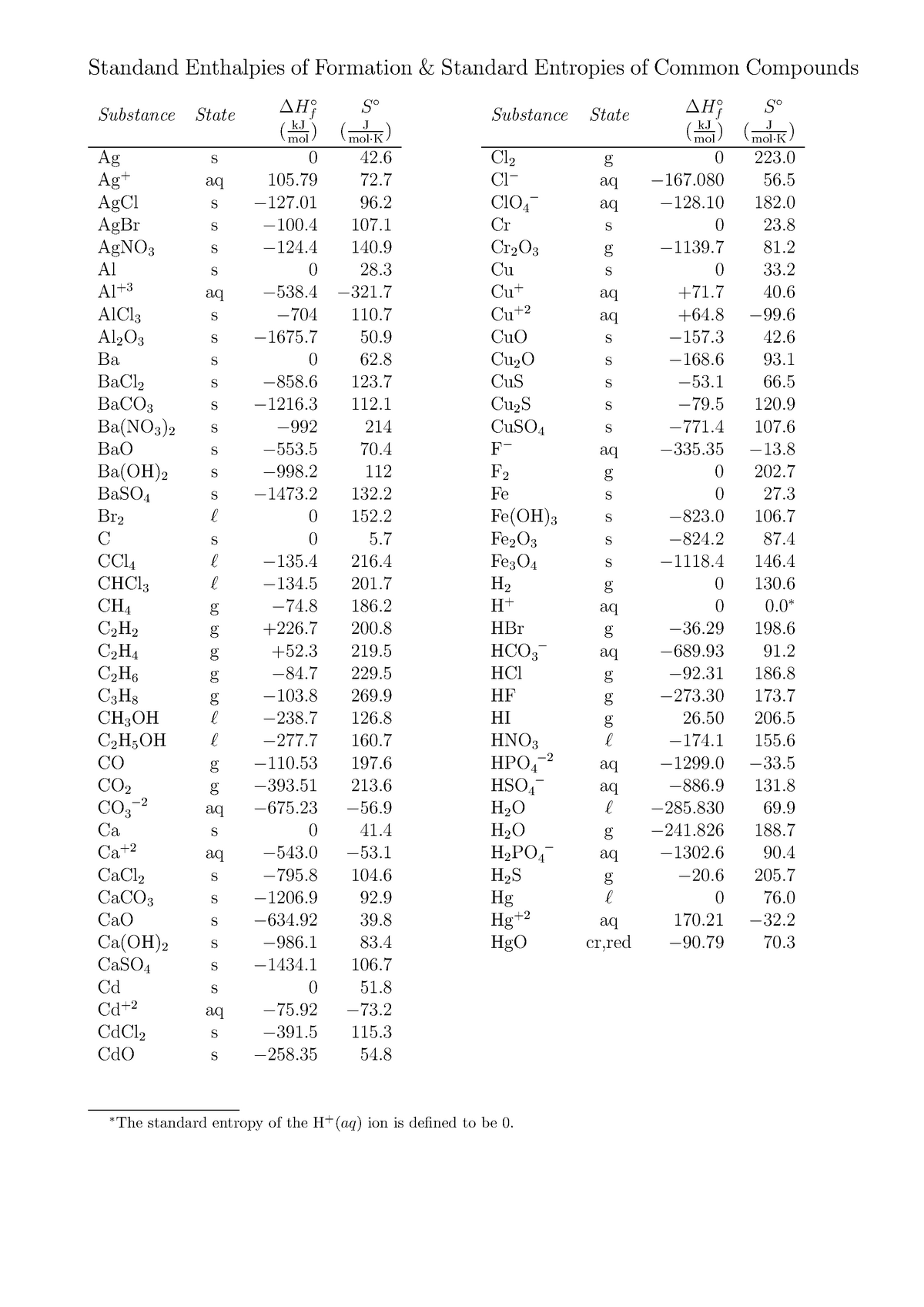
Standard Entropy Of Formation Table
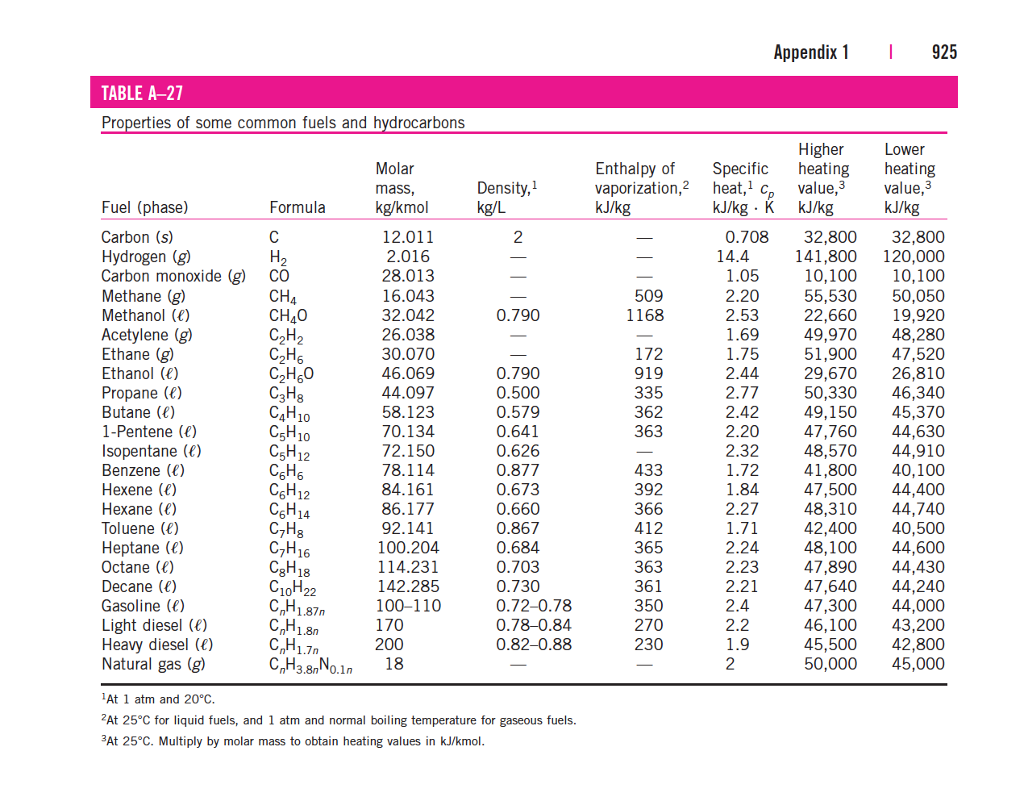
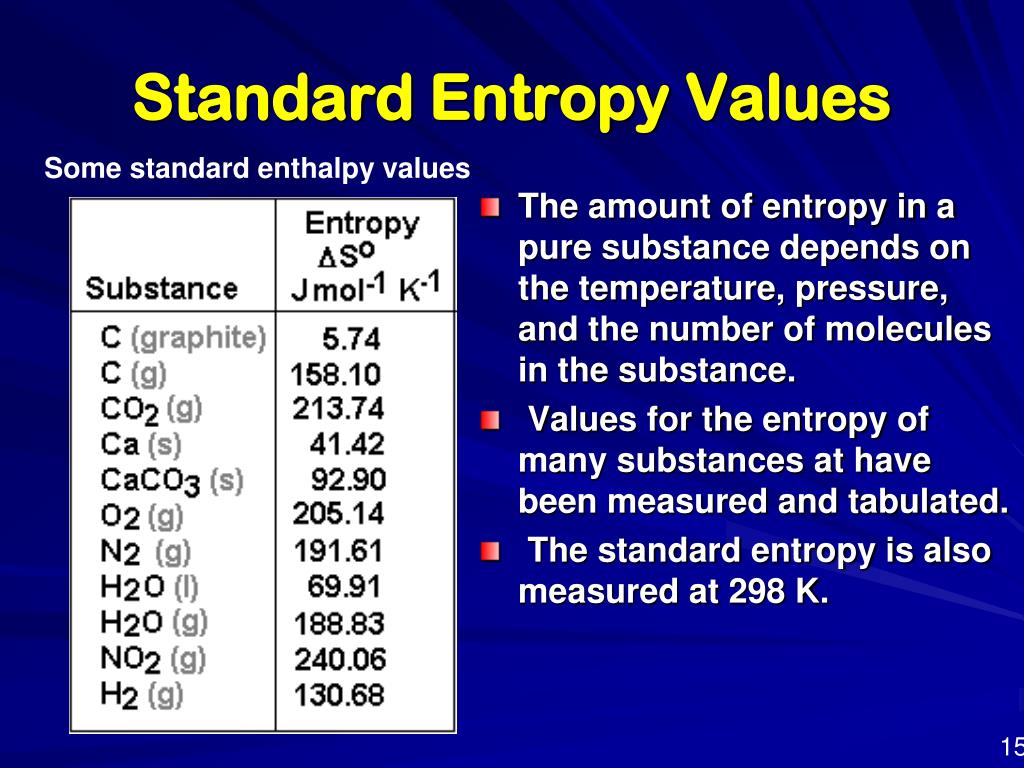
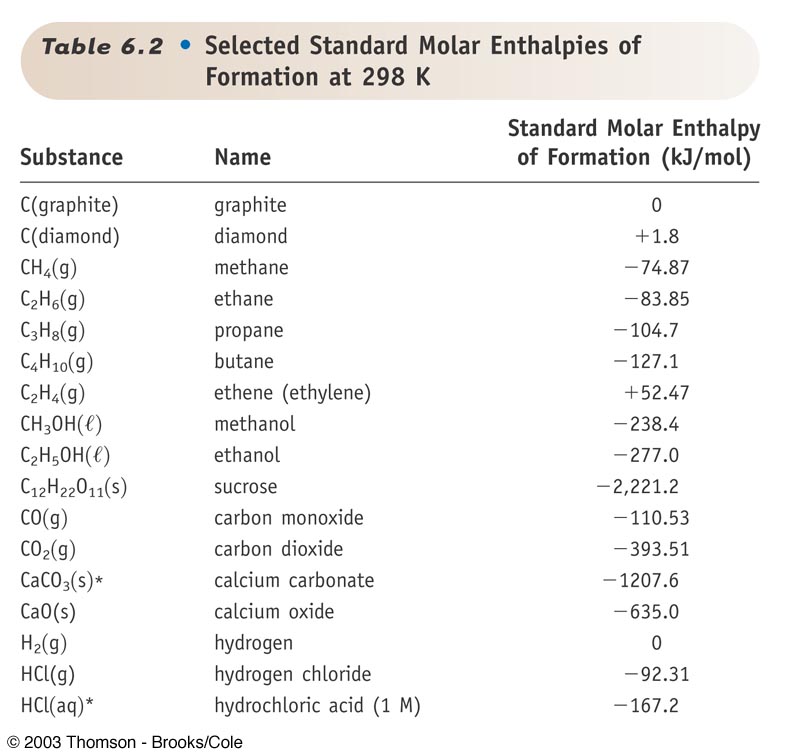
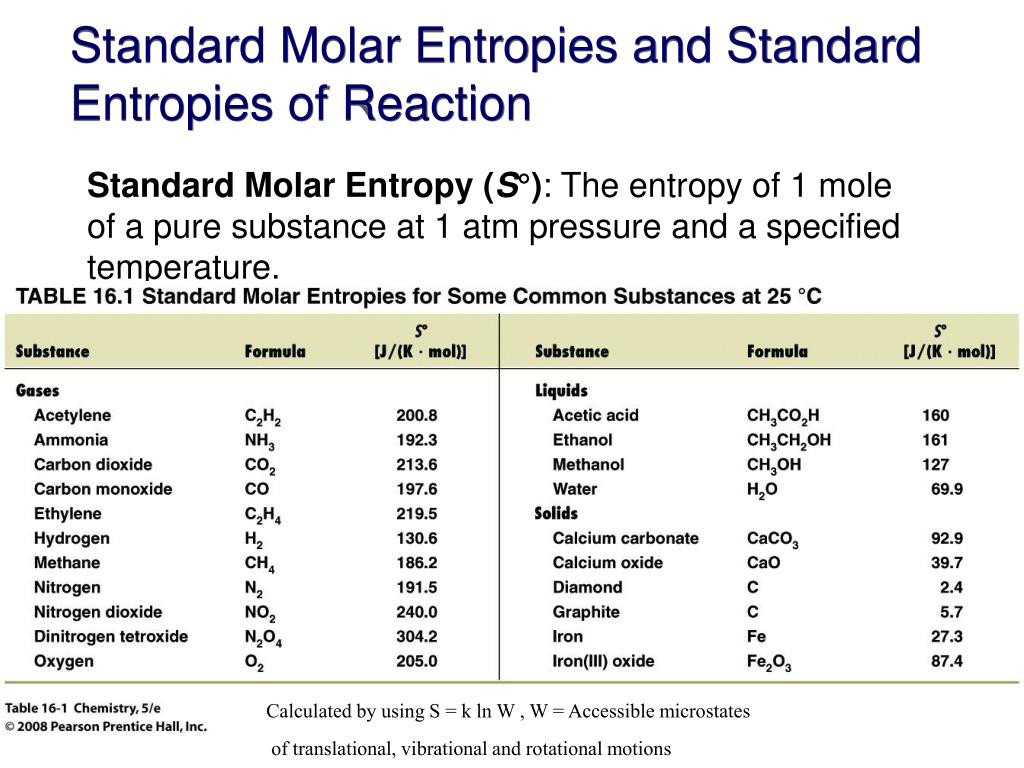
Standard Entropy Of Formation Table - Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class; The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds ∗the standard entropy of the h+(aq) ion is defined to be 0. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the.
∗the standard entropy of the h+(aq) ion is defined to be 0. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class;
100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class; ∗the standard entropy of the h+(aq) ion is defined to be 0.
TABLE A286 Enthalpy of formation, Gibbs function of
Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class; The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. ∗the standard entropy of the h+(aq) ion is defined to be 0. 100 rows standard heats and free energies of formation and absolute entropies of.
Table II from Largescale calculations of gas phase thermochemistry
∗the standard entropy of the h+(aq) ion is defined to be 0. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds Compound state δh f ° (kj/mol) δg f.
1. For the reaction Hg(1) + O2 → HgO(s) Use absolute entropies from
100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds ∗the standard entropy of the h+(aq) ion is defined to be 0. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The table below shows the standard enthalpy of.
Change in Enthalpy Chemistry lessons, Chemistry, Ap chemistry
This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. ∗the standard entropy of the h+(aq) ion is defined to be 0. 100 rows standard heats.
PPT Entropy PowerPoint Presentation, free download ID2017581
The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class; 100 rows.
Astounding Collections Of Heat Of Formation Table Photos Darkata
The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. ∗the standard entropy of the h+(aq) ion is defined to be 0. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds This table lists the standard enthalpies (δh°), the free energies.
Table I from Largescale calculations of gas phase thermochemistry
The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class; ∗the standard.
Heat Of Formation Chart
Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class; ∗the standard entropy of the h+(aq) ion is defined to be 0. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation.
Standard entropy wsmzaer
The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class; 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds This table lists the standard enthalpies (δh°), the.
Standard entropy table Lasialabama
Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class; The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds ∗the standard entropy of the h+(aq) ion is.
The Table Below Shows The Standard Enthalpy Of Formation, The Standard Gibbs Free Energy Of Formation, Standard Entropy And Molar Heat.
∗the standard entropy of the h+(aq) ion is defined to be 0. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Compound state δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) class;