What Are The Expected Bond Angles In Icl4
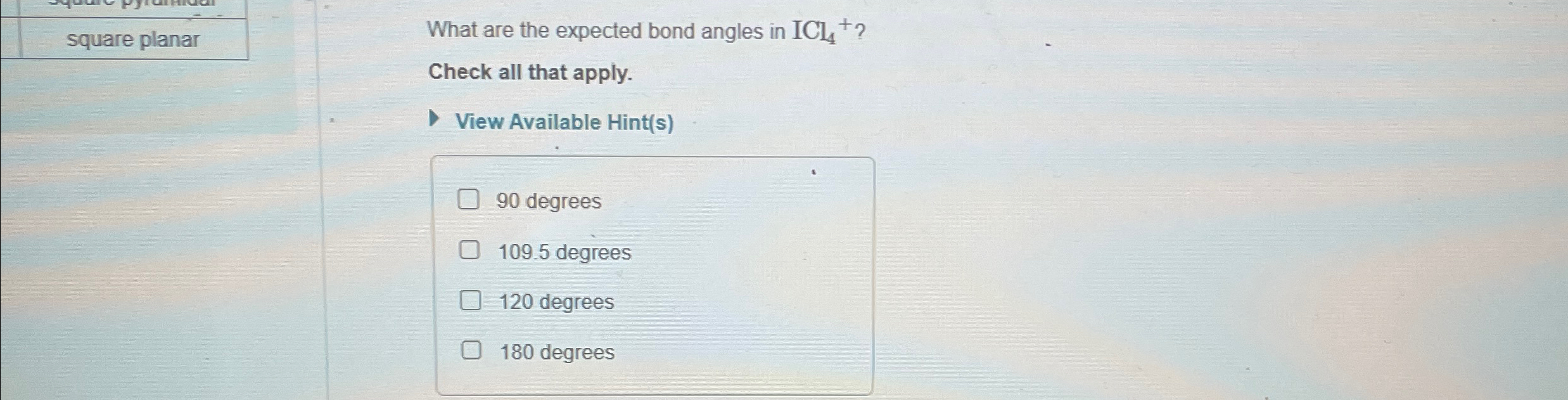
What Are The Expected Bond Angles In Icl4 - What are the expected bond angles in icl4+? The icl4+ ion has a square planar shape due to the presence of two lone pairs of electrons on the central iodine (i) atom. 90 degrees is the expected bond angle in icl₄. The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation. 90 degrees, 109.5 degrees, 180 degrees, 120 degrees Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine.
What are the expected bond angles in icl4+? The icl4+ ion has a square planar shape due to the presence of two lone pairs of electrons on the central iodine (i) atom. 90 degrees is the expected bond angle in icl₄. Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine. 90 degrees, 109.5 degrees, 180 degrees, 120 degrees The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation.
What are the expected bond angles in icl4+? The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation. The icl4+ ion has a square planar shape due to the presence of two lone pairs of electrons on the central iodine (i) atom. 90 degrees, 109.5 degrees, 180 degrees, 120 degrees Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine. 90 degrees is the expected bond angle in icl₄.
square planarWhat are the expected bond angles in
What are the expected bond angles in icl4+? The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation. 90 degrees, 109.5 degrees, 180 degrees, 120 degrees 90 degrees is the expected bond angle in icl₄. Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine.
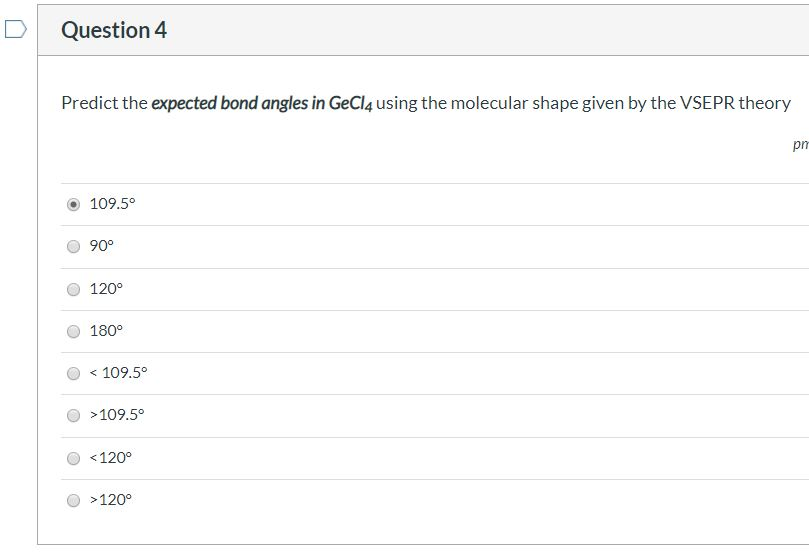
Solved Question 4 Predict the expected bond angles in GeCl4
What are the expected bond angles in icl4+? 90 degrees, 109.5 degrees, 180 degrees, 120 degrees 90 degrees is the expected bond angle in icl₄. Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine. The icl4+ ion has a square planar shape due to the presence of two lone pairs of electrons on the central iodine (i).
Ideal Bond Angles — Overview & Examples Expii
90 degrees is the expected bond angle in icl₄. The icl4+ ion has a square planar shape due to the presence of two lone pairs of electrons on the central iodine (i) atom. The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation. 90 degrees, 109.5 degrees,.
SOLVED What are the expected bond angles in ICl? Check all that apply
Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine. What are the expected bond angles in icl4+? 90 degrees, 109.5 degrees, 180 degrees, 120 degrees 90 degrees is the expected bond angle in icl₄. The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation.
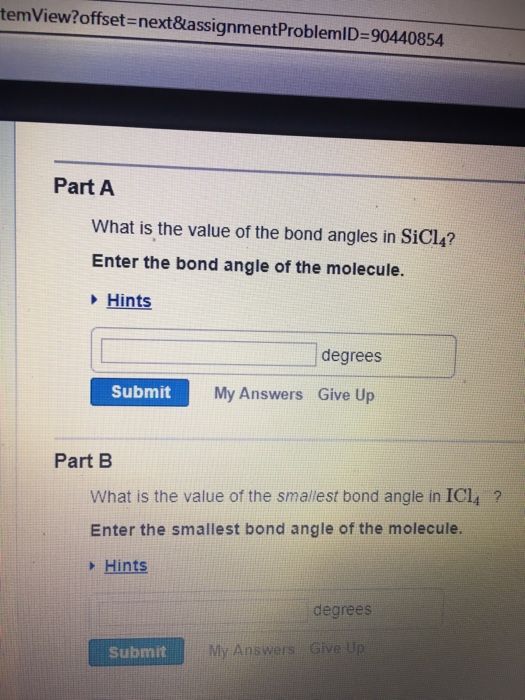
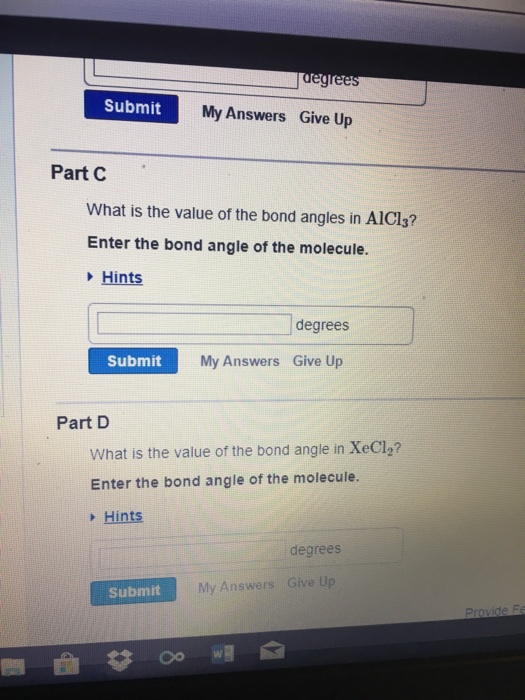
Solved Part A) What is the value of the bond angles in
90 degrees is the expected bond angle in icl₄. Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine. The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation. 90 degrees, 109.5 degrees, 180 degrees, 120 degrees What are the expected bond angles in icl4+?
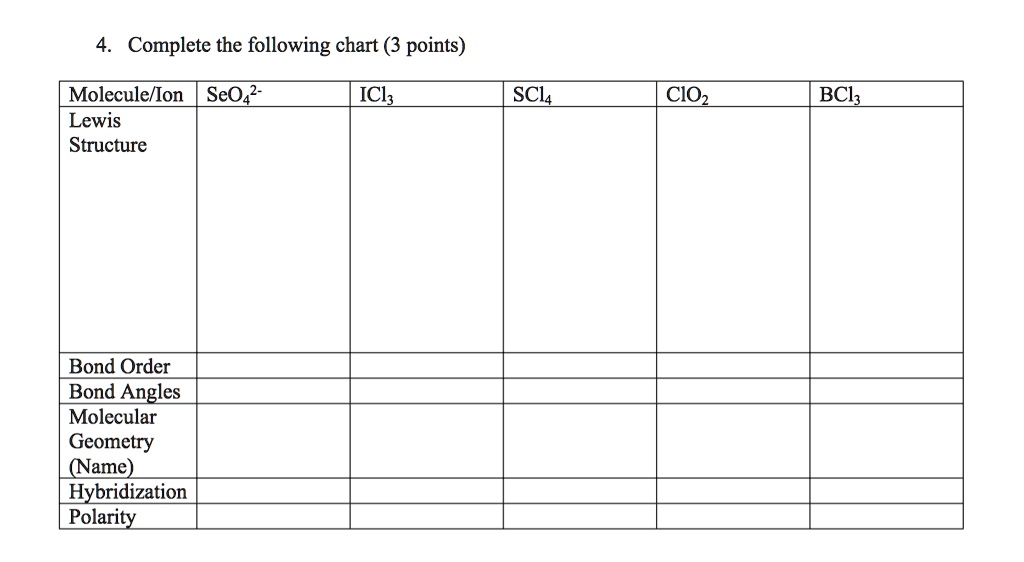
SOLVED Text Complete the following chart (3 points) Molecule/Ion
The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation. 90 degrees is the expected bond angle in icl₄. What are the expected bond angles in icl4+? 90 degrees, 109.5 degrees, 180 degrees, 120 degrees Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine.
Selected bond lengths (Å) and bond angles (°) of HLClO 4 ·H 2 O and CP
90 degrees is the expected bond angle in icl₄. Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine. What are the expected bond angles in icl4+? The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation. 90 degrees, 109.5 degrees, 180 degrees, 120 degrees
Molecular Geometry and Bond Angles Quiz
90 degrees is the expected bond angle in icl₄. The icl4+ ion has a square planar shape due to the presence of two lone pairs of electrons on the central iodine (i) atom. The expected bond angles in icl4+ are 90 degrees and 180 degrees, due to its square planar geometry and the ax4e2 vsepr designation. Iodine tetrachloride has a.
Solved Part A) What is the value of the bond angles in
90 degrees is the expected bond angle in icl₄. The icl4+ ion has a square planar shape due to the presence of two lone pairs of electrons on the central iodine (i) atom. What are the expected bond angles in icl4+? 90 degrees, 109.5 degrees, 180 degrees, 120 degrees Iodine tetrachloride has a central iodine atom (i) bonded to four.
Icl3 Molecular Geometry Bond Angles
What are the expected bond angles in icl4+? 90 degrees, 109.5 degrees, 180 degrees, 120 degrees 90 degrees is the expected bond angle in icl₄. The icl4+ ion has a square planar shape due to the presence of two lone pairs of electrons on the central iodine (i) atom. The expected bond angles in icl4+ are 90 degrees and 180.
The Expected Bond Angles In Icl4+ Are 90 Degrees And 180 Degrees, Due To Its Square Planar Geometry And The Ax4E2 Vsepr Designation.
Iodine tetrachloride has a central iodine atom (i) bonded to four chlorine. 90 degrees is the expected bond angle in icl₄. 90 degrees, 109.5 degrees, 180 degrees, 120 degrees What are the expected bond angles in icl4+?