What Do Cs And Cl React To
What Do Cs And Cl React To - What is a likely oxidation state of chlorine? Cesium as the most reactive metal forms cesium halides as it reacts strongly with all. And the halogen atom ‘chlorine’ stands for the symbol ‘cl’ lies in the group seventeenth of the periodic. What do you think is the reason for an. What do cs and cl react to form? The reaction of cs with chlorine: Symbol ‘cs’ stand for the element ‘caesium ‘, ‘cs’ lies in the group one of the periodic table. How many valence electrons does na have when forming an ionic bond with cl? Cs and cl react to form an ionic compound known as cesium chloride (cscl), where cs becomes a cation and cl becomes an anion held together by ionic bonds. Alkali metals are known to readily react with halogens to form ionic halides.
How many valence electrons does na have when forming an ionic bond with cl? What do cs and cl react to form? Cesium as the most reactive metal forms cesium halides as it reacts strongly with all. Symbol ‘cs’ stand for the element ‘caesium ‘, ‘cs’ lies in the group one of the periodic table. Alkali metals are known to readily react with halogens to form ionic halides. The reaction of cs with chlorine: What do you think is the reason for an. Cs and cl react to form an ionic compound known as cesium chloride (cscl), where cs becomes a cation and cl becomes an anion held together by ionic bonds. What is a likely oxidation state of chlorine? And the halogen atom ‘chlorine’ stands for the symbol ‘cl’ lies in the group seventeenth of the periodic.
Symbol ‘cs’ stand for the element ‘caesium ‘, ‘cs’ lies in the group one of the periodic table. What is a likely oxidation state of chlorine? And the halogen atom ‘chlorine’ stands for the symbol ‘cl’ lies in the group seventeenth of the periodic. Alkali metals are known to readily react with halogens to form ionic halides. What do cs and cl react to form? What do you think is the reason for an. The reaction of cs with chlorine: Cesium as the most reactive metal forms cesium halides as it reacts strongly with all. How many valence electrons does na have when forming an ionic bond with cl? Cs and cl react to form an ionic compound known as cesium chloride (cscl), where cs becomes a cation and cl becomes an anion held together by ionic bonds.
Cs and Cl NMR spectroscopy and molecular dynamics modeling of
Symbol ‘cs’ stand for the element ‘caesium ‘, ‘cs’ lies in the group one of the periodic table. What is a likely oxidation state of chlorine? Cs and cl react to form an ionic compound known as cesium chloride (cscl), where cs becomes a cation and cl becomes an anion held together by ionic bonds. And the halogen atom ‘chlorine’.
CL Biography Facts, Childhood, Family Life & Achievements
The reaction of cs with chlorine: Cs and cl react to form an ionic compound known as cesium chloride (cscl), where cs becomes a cation and cl becomes an anion held together by ionic bonds. Symbol ‘cs’ stand for the element ‘caesium ‘, ‘cs’ lies in the group one of the periodic table. Cesium as the most reactive metal forms.
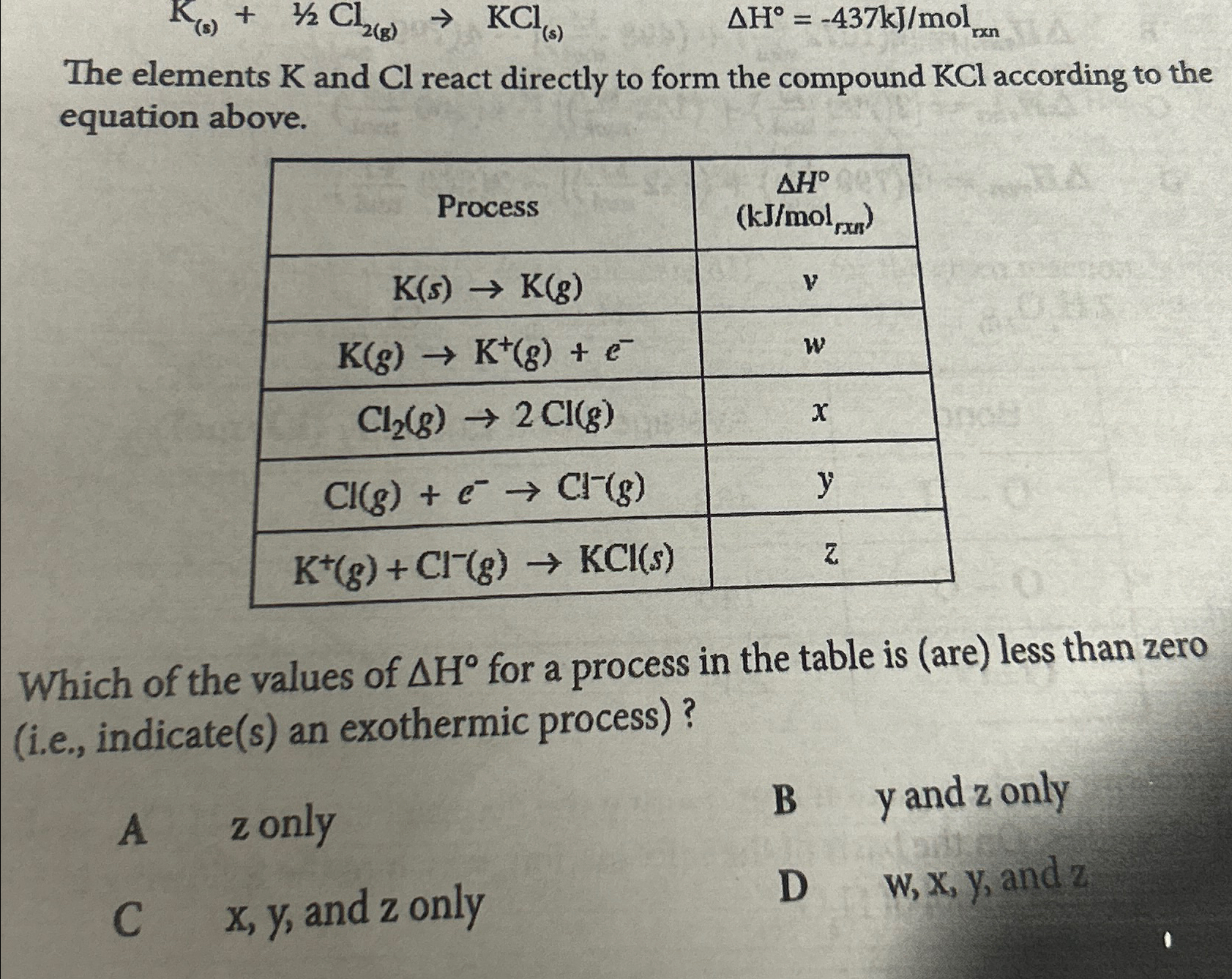
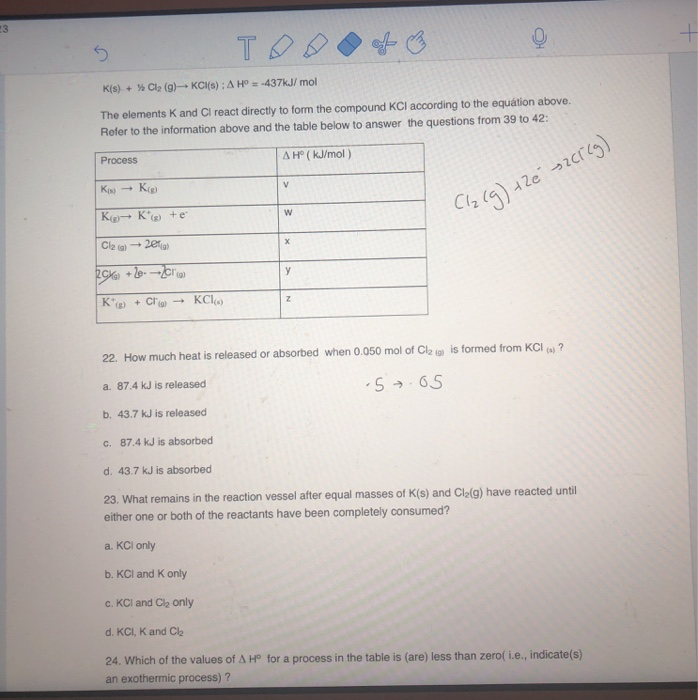
Solved The elements K and Cl react directly to form the
What do cs and cl react to form? The reaction of cs with chlorine: And the halogen atom ‘chlorine’ stands for the symbol ‘cl’ lies in the group seventeenth of the periodic. How many valence electrons does na have when forming an ionic bond with cl? What do you think is the reason for an.
Solved K(s) + Cl (g) KCl(s) A Hº = 437kJ/mol The
What is a likely oxidation state of chlorine? What do cs and cl react to form? Alkali metals are known to readily react with halogens to form ionic halides. And the halogen atom ‘chlorine’ stands for the symbol ‘cl’ lies in the group seventeenth of the periodic. Cs and cl react to form an ionic compound known as cesium chloride.
React Js Olajirebashitsayo Medium
What is a likely oxidation state of chlorine? Cs and cl react to form an ionic compound known as cesium chloride (cscl), where cs becomes a cation and cl becomes an anion held together by ionic bonds. The reaction of cs with chlorine: Symbol ‘cs’ stand for the element ‘caesium ‘, ‘cs’ lies in the group one of the periodic.
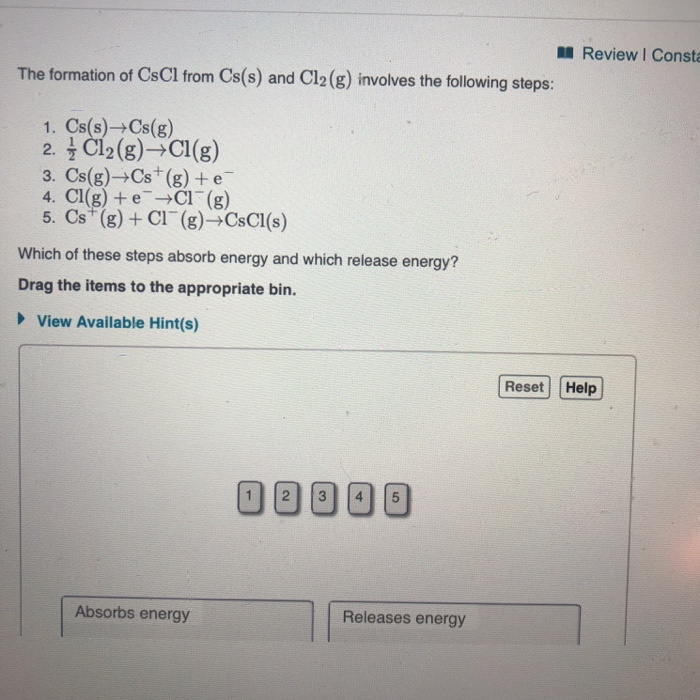
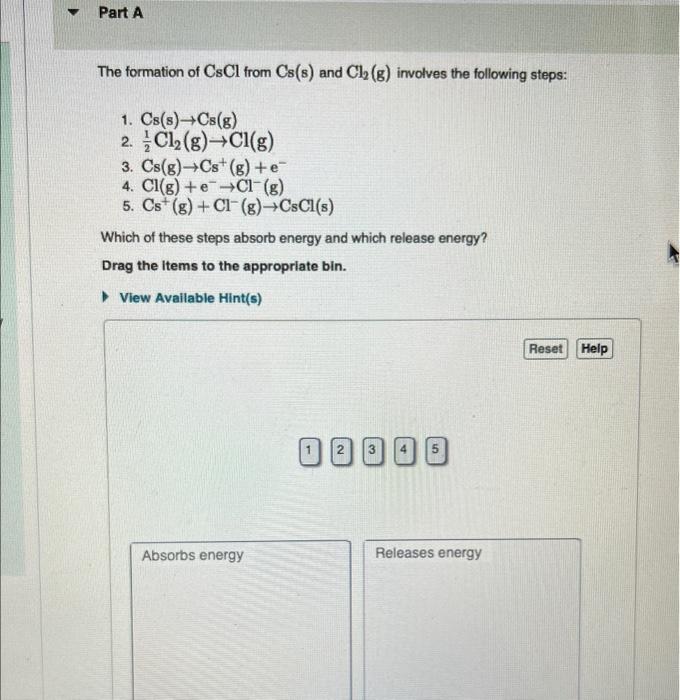
Solved Review I Consta The formation of CsCl from Cs(s) and
What do you think is the reason for an. The reaction of cs with chlorine: How many valence electrons does na have when forming an ionic bond with cl? Cesium as the most reactive metal forms cesium halides as it reacts strongly with all. Alkali metals are known to readily react with halogens to form ionic halides.
Solved The formation of CsCl from Cs(s) and Cl2( g) involves
What do cs and cl react to form? What is a likely oxidation state of chlorine? Symbol ‘cs’ stand for the element ‘caesium ‘, ‘cs’ lies in the group one of the periodic table. Cesium as the most reactive metal forms cesium halides as it reacts strongly with all. What do you think is the reason for an.
CL Biography Facts, Childhood, Family Life & Achievements
Alkali metals are known to readily react with halogens to form ionic halides. And the halogen atom ‘chlorine’ stands for the symbol ‘cl’ lies in the group seventeenth of the periodic. Cesium as the most reactive metal forms cesium halides as it reacts strongly with all. What is a likely oxidation state of chlorine? Cs and cl react to form.
CL REACT YouTube
What is a likely oxidation state of chlorine? And the halogen atom ‘chlorine’ stands for the symbol ‘cl’ lies in the group seventeenth of the periodic. Symbol ‘cs’ stand for the element ‘caesium ‘, ‘cs’ lies in the group one of the periodic table. What do cs and cl react to form? Alkali metals are known to readily react with.
In CsCl type structure, the coordination number of Cs+ and Cl
And the halogen atom ‘chlorine’ stands for the symbol ‘cl’ lies in the group seventeenth of the periodic. How many valence electrons does na have when forming an ionic bond with cl? Symbol ‘cs’ stand for the element ‘caesium ‘, ‘cs’ lies in the group one of the periodic table. What do cs and cl react to form? The reaction.
Symbol ‘Cs’ Stand For The Element ‘Caesium ‘, ‘Cs’ Lies In The Group One Of The Periodic Table.
How many valence electrons does na have when forming an ionic bond with cl? Cs and cl react to form an ionic compound known as cesium chloride (cscl), where cs becomes a cation and cl becomes an anion held together by ionic bonds. And the halogen atom ‘chlorine’ stands for the symbol ‘cl’ lies in the group seventeenth of the periodic. What do you think is the reason for an.
What Is A Likely Oxidation State Of Chlorine?
The reaction of cs with chlorine: Cesium as the most reactive metal forms cesium halides as it reacts strongly with all. What do cs and cl react to form? Alkali metals are known to readily react with halogens to form ionic halides.