What Element Has The Largest Atomic Radius
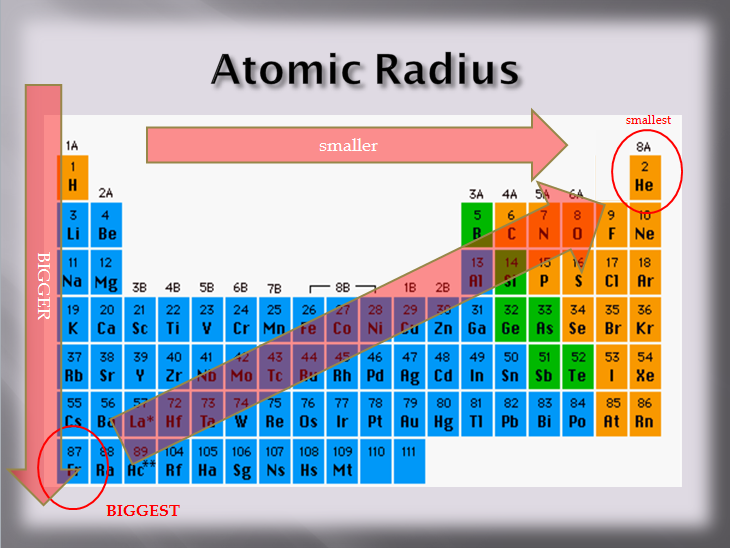
What Element Has The Largest Atomic Radius - Atomic size increases across a period from left to right as we face the table, and increases down a group. Likewise, bromine will have a larger atomic radius when compared with chlorine. But as a chemist, as a. As you know, atomic radii decreases across a period, from left to right as we face the table, and increases down a group. With these trends as the basis, francium occurring in group i and period 7 is the element. And thus the largest atomic radius should be exhibited by the alkali. This results in the greater atomic size and hence increasing atomic radius as we go down a group. Atomic radius increases as you go to the left and downward due to the attraction of electrons and the nucleus in an. Francium has the largest, helium has the lowest. Francium would be a good bet.
Atomic radius increases as you go to the left and downward due to the attraction of electrons and the nucleus in an. Francium would be a good bet. Francium has the largest, helium has the lowest. And thus the largest atomic radius should be exhibited by the alkali. Likewise, bromine will have a larger atomic radius when compared with chlorine. This results in the greater atomic size and hence increasing atomic radius as we go down a group. As you know, atomic radii decreases across a period, from left to right as we face the table, and increases down a group. Atomic size increases across a period from left to right as we face the table, and increases down a group. Now focus on potassium and bromine. Since potassium is located at the start of period 3, and bromine.
But as a chemist, as a. Since potassium is located at the start of period 3, and bromine. Francium would be a good bet. Atomic size increases across a period from left to right as we face the table, and increases down a group. And thus the largest atomic radius should be exhibited by the alkali. Now focus on potassium and bromine. Francium has the largest, helium has the lowest. Likewise, bromine will have a larger atomic radius when compared with chlorine. As you know, atomic radii decreases across a period, from left to right as we face the table, and increases down a group. Atomic radius increases as you go to the left and downward due to the attraction of electrons and the nucleus in an.
LIST OF ATOMIC RADIUS AND ATOMIC WEIGHTS OF ELEMENTS BASIC INFORMATION
Atomic radius increases as you go to the left and downward due to the attraction of electrons and the nucleus in an. Since potassium is located at the start of period 3, and bromine. Atomic size increases across a period from left to right as we face the table, and increases down a group. This results in the greater atomic.
Largest Atomic Radius Periodic Table Periodic Table Timeline
Francium would be a good bet. And thus the largest atomic radius should be exhibited by the alkali. With these trends as the basis, francium occurring in group i and period 7 is the element. Since potassium is located at the start of period 3, and bromine. This results in the greater atomic size and hence increasing atomic radius as.
Atomic Radius Periodic Table NEET Lab
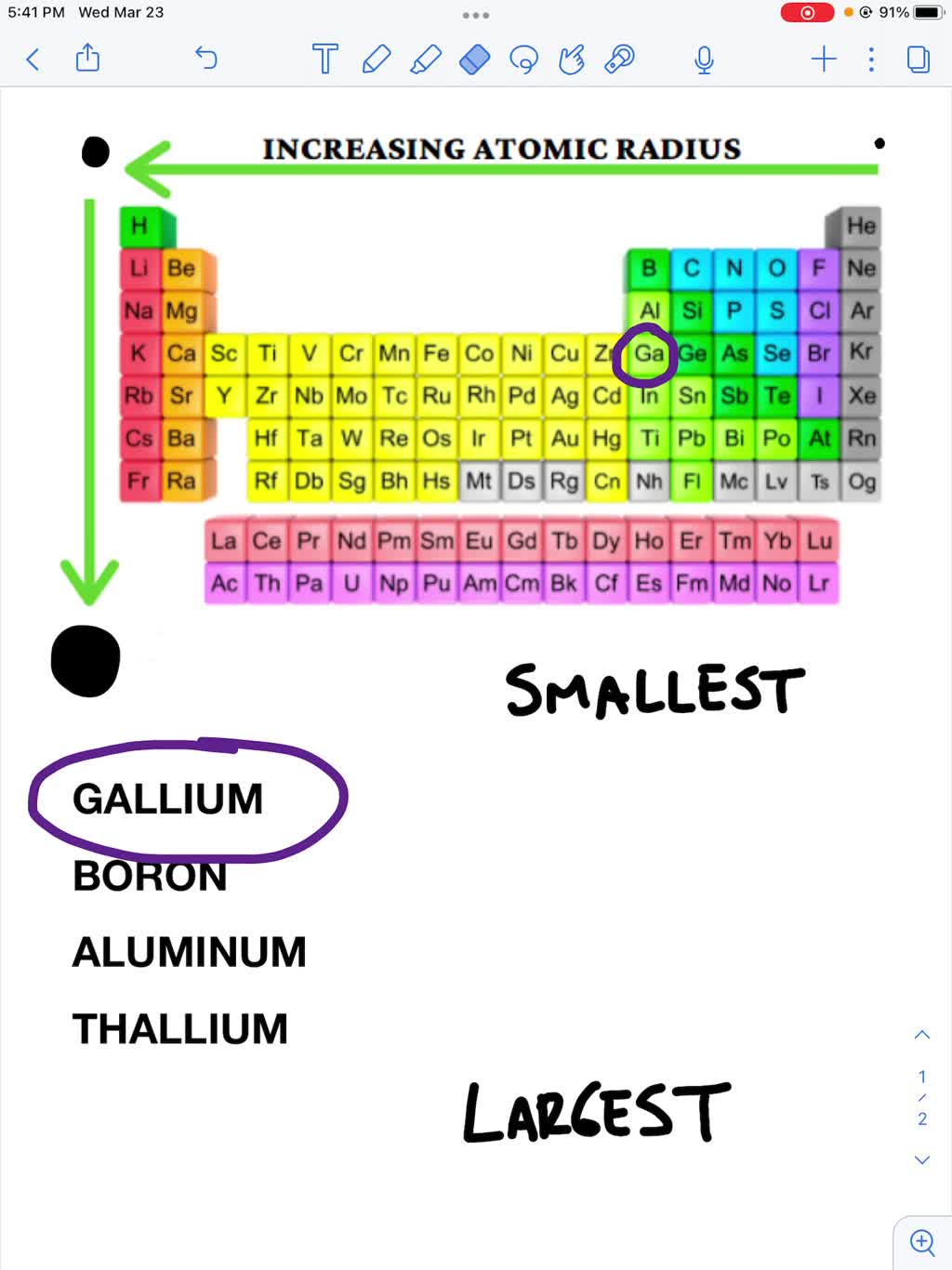
And thus the largest atomic radius should be exhibited by the alkali. Atomic size increases across a period from left to right as we face the table, and increases down a group. Now focus on potassium and bromine. As you know, atomic radii decreases across a period, from left to right as we face the table, and increases down a.
Largest Atomic Radius Periodic Table Elcho Table
And thus the largest atomic radius should be exhibited by the alkali. Francium has the largest, helium has the lowest. Atomic size increases across a period from left to right as we face the table, and increases down a group. Francium would be a good bet. This results in the greater atomic size and hence increasing atomic radius as we.
Which Element Has The Largest Atomic Radius Jacks Of Science
Now focus on potassium and bromine. This results in the greater atomic size and hence increasing atomic radius as we go down a group. With these trends as the basis, francium occurring in group i and period 7 is the element. Francium has the largest, helium has the lowest. Atomic size increases across a period from left to right as.
Periodic Table Largest Atomic Radius Elcho Table
Atomic size increases across a period from left to right as we face the table, and increases down a group. And thus the largest atomic radius should be exhibited by the alkali. This results in the greater atomic size and hence increasing atomic radius as we go down a group. Likewise, bromine will have a larger atomic radius when compared.
Which Element Has The Largest Atomic Radius Jacks Of Science
This results in the greater atomic size and hence increasing atomic radius as we go down a group. Atomic radius increases as you go to the left and downward due to the attraction of electrons and the nucleus in an. As you know, atomic radii decreases across a period, from left to right as we face the table, and increases.
Largest Atomic Radius Periodic Table Elcho Table
And thus the largest atomic radius should be exhibited by the alkali. Since potassium is located at the start of period 3, and bromine. Francium has the largest, helium has the lowest. But as a chemist, as a. As you know, atomic radii decreases across a period, from left to right as we face the table, and increases down a.
Which atom has the largest atomic radius lucidstart
Atomic size increases across a period from left to right as we face the table, and increases down a group. Francium has the largest, helium has the lowest. And thus the largest atomic radius should be exhibited by the alkali. Atomic radius increases as you go to the left and downward due to the attraction of electrons and the nucleus.
Which Element On The Periodic Table Has Highest Atomic Radius
And thus the largest atomic radius should be exhibited by the alkali. But as a chemist, as a. Francium has the largest, helium has the lowest. As you know, atomic radii decreases across a period, from left to right as we face the table, and increases down a group. With these trends as the basis, francium occurring in group i.
Francium Would Be A Good Bet.
This results in the greater atomic size and hence increasing atomic radius as we go down a group. But as a chemist, as a. As you know, atomic radii decreases across a period, from left to right as we face the table, and increases down a group. Since potassium is located at the start of period 3, and bromine.
Now Focus On Potassium And Bromine.
Atomic size increases across a period from left to right as we face the table, and increases down a group. Atomic radius increases as you go to the left and downward due to the attraction of electrons and the nucleus in an. With these trends as the basis, francium occurring in group i and period 7 is the element. Francium has the largest, helium has the lowest.
And Thus The Largest Atomic Radius Should Be Exhibited By The Alkali.
Likewise, bromine will have a larger atomic radius when compared with chlorine.