What Happens To An Atom When It Loses An Electron
What Happens To An Atom When It Loses An Electron - Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. Atoms can gain or lose electrons to become ions. Answer:when an atom loses electron (s) it will lose some of its negative charge and so becomes positively charged. When an atom loses or gains an electron, it undergoes a process called ionization, resulting in the formation of an ion. Atoms and chemical species lose or gain electrons when they react in order to gain stability. Thus, typically, metals (with nearly. If an atom or molecule gains an. When an atom loses an electron, it undergoes a change in charge. When an atom loses an electron it gains a positive charge and is called a cation. Let's break down why this happens:
Answer:when an atom loses electron (s) it will lose some of its negative charge and so becomes positively charged. When an atom loses or gains an electron, it undergoes a process called ionization, resulting in the formation of an ion. Atoms can gain or lose electrons to become ions. If an atom or molecule gains an. Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. Atoms and chemical species lose or gain electrons when they react in order to gain stability. Let's break down why this happens: Thus, typically, metals (with nearly. When an atom loses an electron, it undergoes a change in charge. When an atom loses an electron it gains a positive charge and is called a cation.
Atoms can gain or lose electrons to become ions. Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. Answer:when an atom loses electron (s) it will lose some of its negative charge and so becomes positively charged. When an atom loses an electron, it undergoes a change in charge. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Let's break down why this happens: If an atom or molecule gains an. When an atom loses or gains an electron, it undergoes a process called ionization, resulting in the formation of an ion. When an atom loses an electron it gains a positive charge and is called a cation. Atoms and chemical species lose or gain electrons when they react in order to gain stability.
WHAT HAPPENS IF YOU SPLIT AN ATOM? LikeFigures
Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Atoms can gain or lose electrons to become ions. When an atom loses an electron it gains a positive charge and is called a cation. When an atom loses or gains an electron, it undergoes a process.
SOLVED What happens to an atom of sulfur (S) if it loses an electron
Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. When an atom loses an electron it gains a positive charge and is called a cation. If an atom or molecule gains an. When an atom loses an electron, it undergoes a change in charge. Thus, typically,.
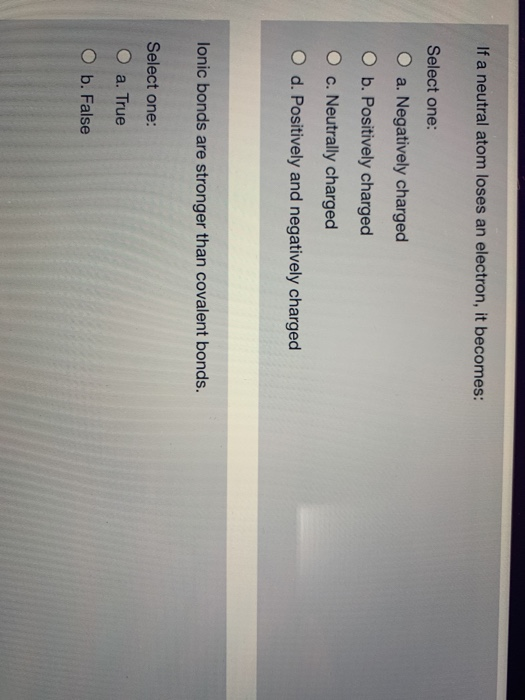
Solved If a neutral atom loses an electron, it
If an atom or molecule gains an. Let's break down why this happens: Atoms and chemical species lose or gain electrons when they react in order to gain stability. When an atom loses an electron, it undergoes a change in charge. Ionization is the process by which ions are formed by gain or loss of an electron from an atom.
Solved If an atom loses an electron, it is (a) negatively
When an atom loses an electron, it undergoes a change in charge. Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. Atoms and chemical species lose or gain electrons when they react in order to gain stability. Atoms tend to lose, gain, or share some valance electrons, making.
16 What happens when an atom gains or loses electrons 16 What
Let's break down why this happens: Atoms can gain or lose electrons to become ions. When an atom loses an electron, it undergoes a change in charge. If an atom or molecule gains an. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,.
When an Atom Loses an Electron What Is Its Charge What is the charge
When an atom loses an electron it gains a positive charge and is called a cation. Thus, typically, metals (with nearly. When an atom loses or gains an electron, it undergoes a process called ionization, resulting in the formation of an ion. Answer:when an atom loses electron (s) it will lose some of its negative charge and so becomes positively.
an atom loses an electron. he says i really gotta keep an ion them
When an atom loses or gains an electron, it undergoes a process called ionization, resulting in the formation of an ion. Atoms can gain or lose electrons to become ions. Answer:when an atom loses electron (s) it will lose some of its negative charge and so becomes positively charged. Let's break down why this happens: Atoms tend to lose, gain,.
Solved What happens to an electron in an atom when it
When an atom loses an electron it gains a positive charge and is called a cation. If an atom or molecule gains an. When an atom loses an electron, it undergoes a change in charge. Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. Thus, typically, metals (with.
When an atom loses an electron, it MakeTheBrainHappy
Answer:when an atom loses electron (s) it will lose some of its negative charge and so becomes positively charged. Let's break down why this happens: Atoms and chemical species lose or gain electrons when they react in order to gain stability. Ionization is the process by which ions are formed by gain or loss of an electron from an atom.
WHAT HAPPENS IF YOU SPLIT AN ATOM mousetimes
Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. Thus, typically, metals (with nearly. If an atom or molecule gains an. When an atom loses an electron, it undergoes a change in charge. Atoms and chemical species lose or gain electrons when they react in order to gain.
Let's Break Down Why This Happens:
Answer:when an atom loses electron (s) it will lose some of its negative charge and so becomes positively charged. When an atom loses an electron, it undergoes a change in charge. When an atom loses or gains an electron, it undergoes a process called ionization, resulting in the formation of an ion. When an atom loses an electron it gains a positive charge and is called a cation.
Ionization Is The Process By Which Ions Are Formed By Gain Or Loss Of An Electron From An Atom Or Molecule.
Atoms can gain or lose electrons to become ions. Thus, typically, metals (with nearly. If an atom or molecule gains an. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,.