What Happens To Red Blood Cells In Distilled Water
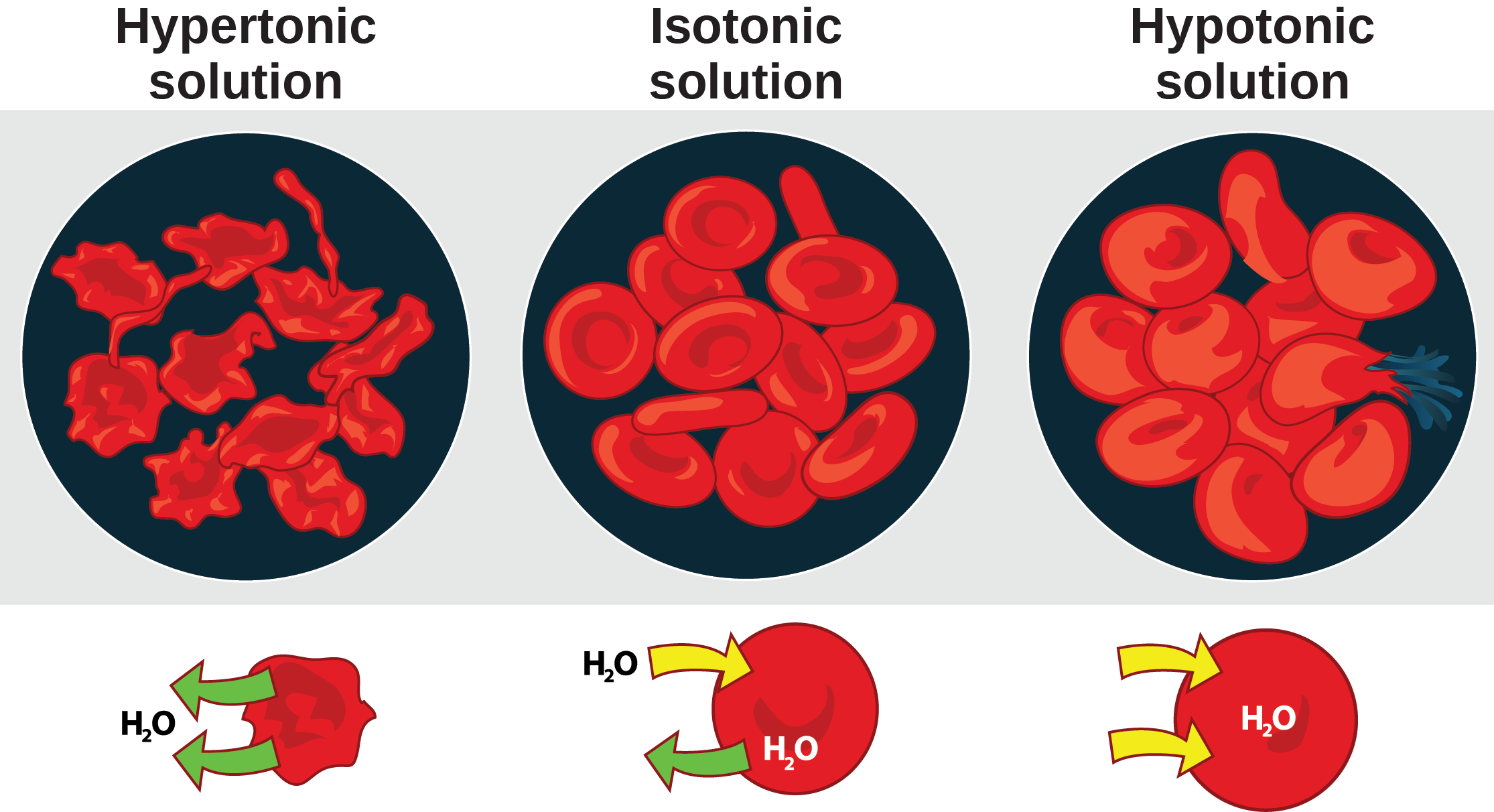
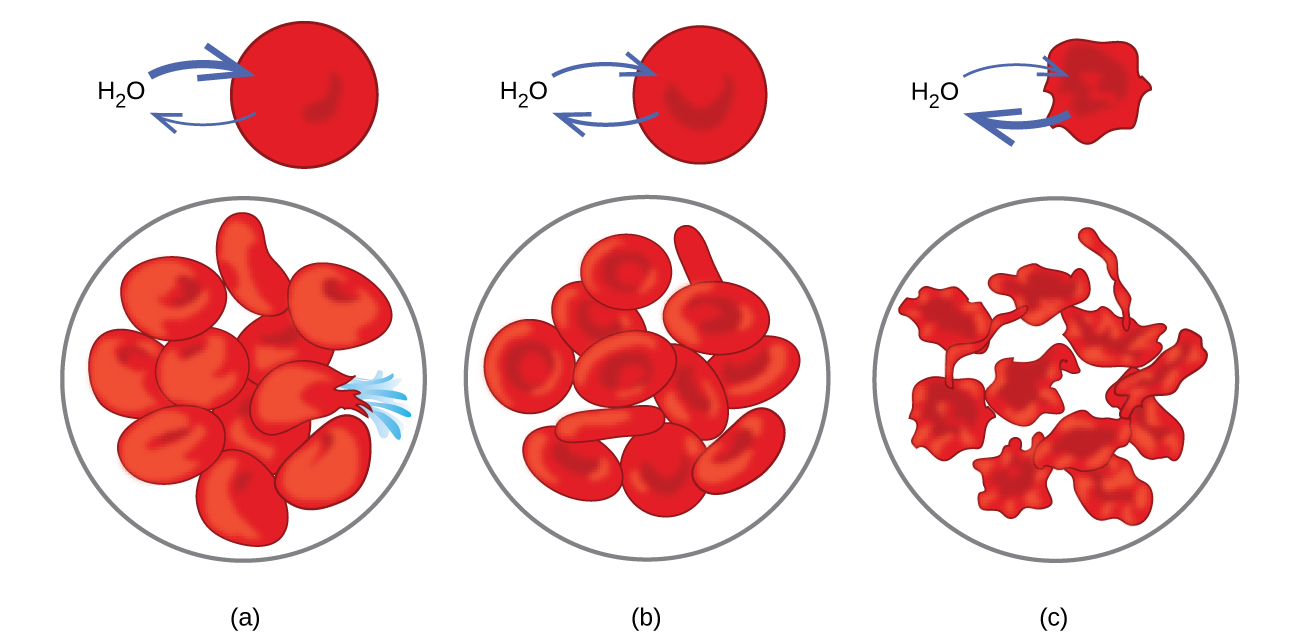
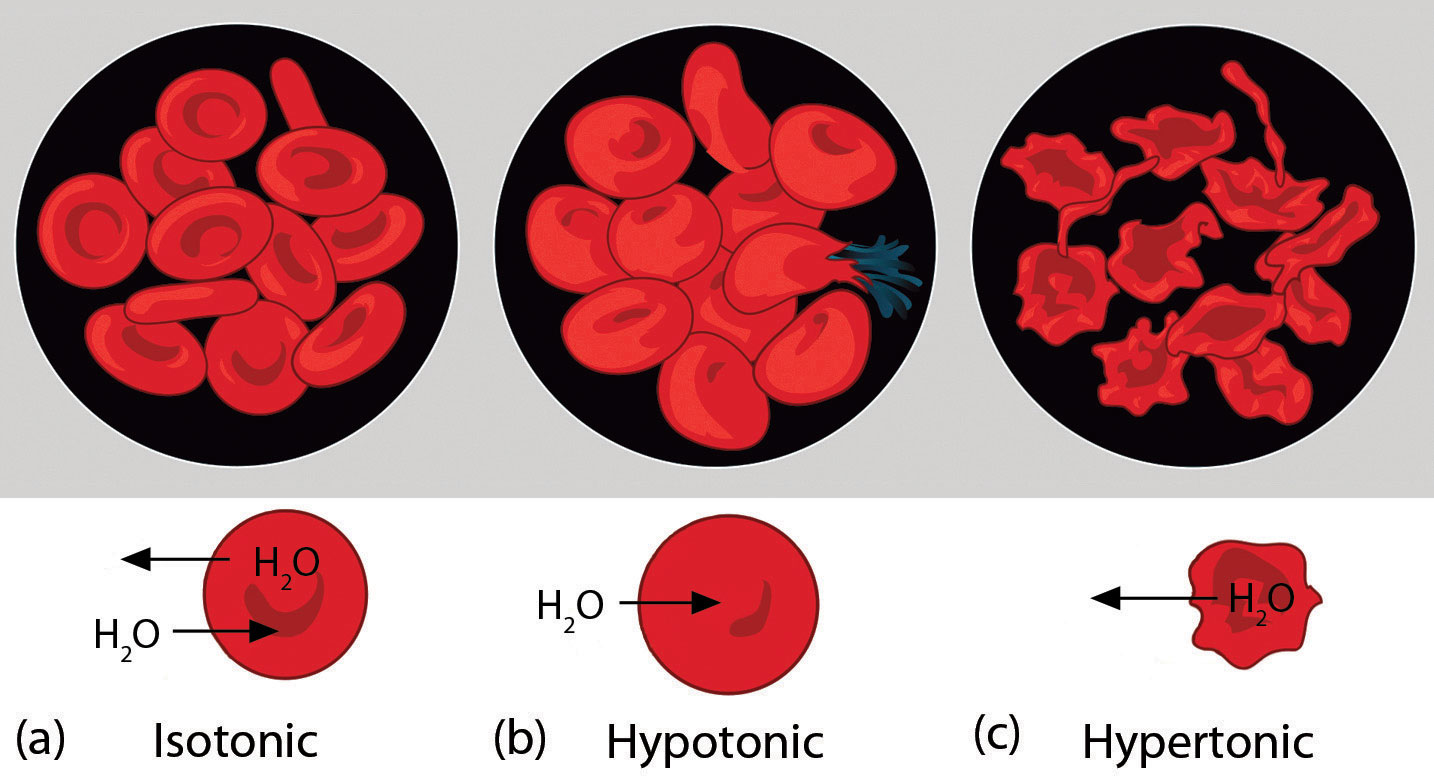
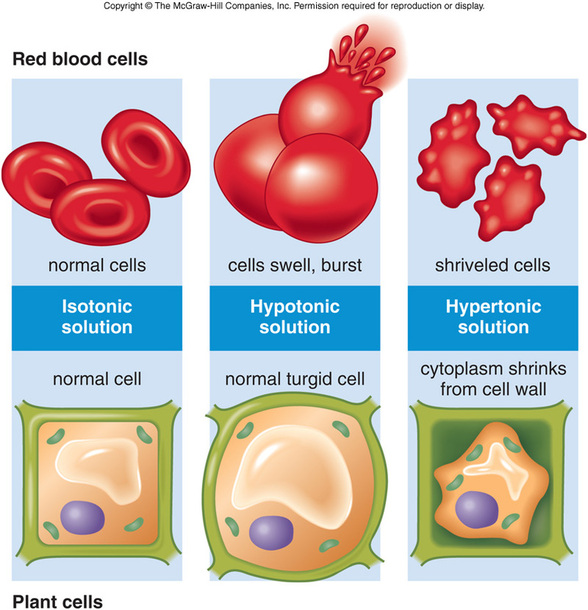
What Happens To Red Blood Cells In Distilled Water - Explanation 0.9% saline solution is isotonic compared to your red blood cells (rbcs) and distilled water is hypotonic to your rbcs. When red blood cells are placed in distilled water, which is hypotonic compared to the solution contained within the cells’. Assertion :red blood cells burst when placed in. What happens when a red blood cell is placed inside a hypotonic solution ? When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. Normal rbcs have been shown to. Red blood cells placed in a solution with a lower water concentration compared to their contents (eg 1.7 per cent salt solution) will. Red blood cells (rbcs) are highly differentiated cells, lacking all cell organelles, including the nucleus.
Red blood cells (rbcs) are highly differentiated cells, lacking all cell organelles, including the nucleus. When red blood cells are placed in distilled water, which is hypotonic compared to the solution contained within the cells’. When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. Red blood cells placed in a solution with a lower water concentration compared to their contents (eg 1.7 per cent salt solution) will. What happens when a red blood cell is placed inside a hypotonic solution ? Explanation 0.9% saline solution is isotonic compared to your red blood cells (rbcs) and distilled water is hypotonic to your rbcs. Normal rbcs have been shown to. Assertion :red blood cells burst when placed in.
Explanation 0.9% saline solution is isotonic compared to your red blood cells (rbcs) and distilled water is hypotonic to your rbcs. When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. What happens when a red blood cell is placed inside a hypotonic solution ? Red blood cells (rbcs) are highly differentiated cells, lacking all cell organelles, including the nucleus. When red blood cells are placed in distilled water, which is hypotonic compared to the solution contained within the cells’. Assertion :red blood cells burst when placed in. Red blood cells placed in a solution with a lower water concentration compared to their contents (eg 1.7 per cent salt solution) will. Normal rbcs have been shown to.
What Happens To Red Blood Cells Placed In Distilled Water
Assertion :red blood cells burst when placed in. What happens when a red blood cell is placed inside a hypotonic solution ? When red blood cells are placed in distilled water, which is hypotonic compared to the solution contained within the cells’. When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing.
Osmoregulation and Osmotic Balance OpenStax Biology 2e
What happens when a red blood cell is placed inside a hypotonic solution ? Explanation 0.9% saline solution is isotonic compared to your red blood cells (rbcs) and distilled water is hypotonic to your rbcs. When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. Assertion :red blood cells.
Can You Drink Distilled Water? The Risks of Drinking Distilled Water
Normal rbcs have been shown to. Red blood cells (rbcs) are highly differentiated cells, lacking all cell organelles, including the nucleus. Explanation 0.9% saline solution is isotonic compared to your red blood cells (rbcs) and distilled water is hypotonic to your rbcs. What happens when a red blood cell is placed inside a hypotonic solution ? When red blood cells.
Colligative Properties · Chemistry
When red blood cells are placed in distilled water, which is hypotonic compared to the solution contained within the cells’. Assertion :red blood cells burst when placed in. What happens when a red blood cell is placed inside a hypotonic solution ? When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing.
What Happens To Red Blood Cells Placed In Distilled Water
Red blood cells (rbcs) are highly differentiated cells, lacking all cell organelles, including the nucleus. What happens when a red blood cell is placed inside a hypotonic solution ? When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. Assertion :red blood cells burst when placed in. Red blood.
Chapter 10 Solutions CHE 110 Introduction to Chemistry (Miles
When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. Assertion :red blood cells burst when placed in. What happens when a red blood cell is placed inside a hypotonic solution ? When red blood cells are placed in distilled water, which is hypotonic compared to the solution contained.
Osmosis Notes Biology Mrs.
What happens when a red blood cell is placed inside a hypotonic solution ? Normal rbcs have been shown to. When red blood cells are placed in distilled water, which is hypotonic compared to the solution contained within the cells’. Red blood cells placed in a solution with a lower water concentration compared to their contents (eg 1.7 per cent.
SCB 115 Lab 5 Diffusion and Osmosis Natural Sciences Open Educational
Normal rbcs have been shown to. When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. Red blood cells (rbcs) are highly differentiated cells, lacking all cell organelles, including the nucleus. What happens when a red blood cell is placed inside a hypotonic solution ? Red blood cells placed.
CH3Cells Principle of osmosis applied to red blood cells Diagram
Normal rbcs have been shown to. Red blood cells placed in a solution with a lower water concentration compared to their contents (eg 1.7 per cent salt solution) will. When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. What happens when a red blood cell is placed inside.
What Happens To Red Blood Cells In Distilled Water
Normal rbcs have been shown to. Red blood cells (rbcs) are highly differentiated cells, lacking all cell organelles, including the nucleus. What happens when a red blood cell is placed inside a hypotonic solution ? When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. Red blood cells placed.
Red Blood Cells Placed In A Solution With A Lower Water Concentration Compared To Their Contents (Eg 1.7 Per Cent Salt Solution) Will.
When red blood cells are placed in a hypertonic solution, the higher effective osmotic pressure of the bathing solution compared with. Assertion :red blood cells burst when placed in. Normal rbcs have been shown to. What happens when a red blood cell is placed inside a hypotonic solution ?
When Red Blood Cells Are Placed In Distilled Water, Which Is Hypotonic Compared To The Solution Contained Within The Cells’.
Explanation 0.9% saline solution is isotonic compared to your red blood cells (rbcs) and distilled water is hypotonic to your rbcs. Red blood cells (rbcs) are highly differentiated cells, lacking all cell organelles, including the nucleus.