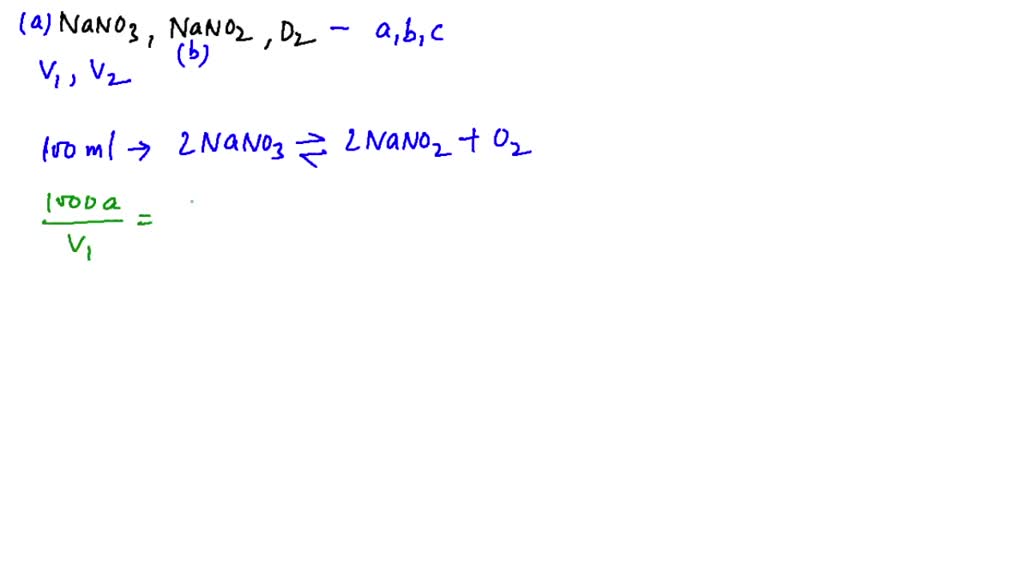What Happens When Nano3 Is Heated
What Happens When Nano3 Is Heated - On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. The chemical formula for sodium nitrate is nano3; Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the decomposition reaction and results in liberating oxygen $. This reaction takes place at a temperature of. When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,.
Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the decomposition reaction and results in liberating oxygen $. This reaction takes place at a temperature of. On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. The chemical formula for sodium nitrate is nano3; When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen.
Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. This reaction takes place at a temperature of. When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. The chemical formula for sodium nitrate is nano3; Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the decomposition reaction and results in liberating oxygen $. On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite.
When NaNO3 is heated in a closed vessel, oxygen is liberated and NaNO2
When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. The chemical formula for sodium nitrate is nano3; Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the decomposition reaction and results in liberating oxygen $. Sodium nitrate undergoes.
When NaNO3 (s) is heated in closed vessel, oxygen is liberated and
When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. This reaction takes place at a temperature of. The chemical formula for sodium nitrate is nano3;
When NaNO3 is heated in a closed vessel, oxygen is liberated and NaNO2
On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. The chemical formula for sodium nitrate is nano3; Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the.
entile When NaNO3 is heated in a closed vessel, oxygen is liberated and
On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the decomposition reaction and results in liberating oxygen $. The chemical formula for sodium nitrate is nano3; Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. When sodium nitrate is.
What Happens When Metal Is Heated? MetalProfy
Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. This reaction takes place at a temperature of. When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the.
SOLVEDWhen NaNO3( d=2.0 g / cc) is heated in a closed vessel of 100 ml
On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the decomposition reaction and results in liberating oxygen $. Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen..
What Happens When Copper Is Heated In Air?
Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. The chemical formula for sodium nitrate is nano3; Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the decomposition reaction and results in liberating oxygen $. This reaction takes place at a temperature of.
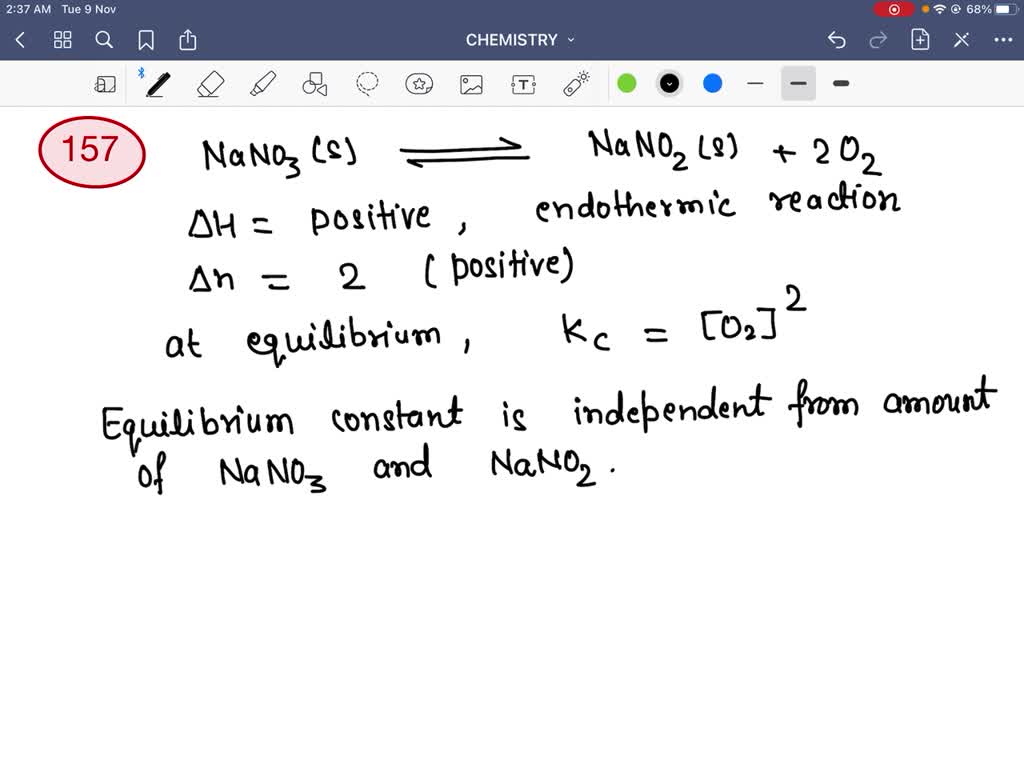
SOLVEDWhen solid NaNO3 is heated in a closed vessel, O2 is liberated
On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. The chemical formula for sodium nitrate is nano3; When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. This reaction takes place at a temperature of.
entile When NaNO3 is heated in a closed vessel, oxygen is liberated and
The chemical formula for sodium nitrate is nano3; Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. This reaction takes place at a temperature of. On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,.
When NaNO3 is heated in a closed vessel, oxygen is liberated and NaNO2
When sodium nitrate is heated, it decomposes to form sodium nitrite, nano2,. This reaction takes place at a temperature of. Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite. The chemical formula for sodium nitrate is nano3;
The Chemical Formula For Sodium Nitrate Is Nano3;
Hence, on heating the compound sodium nitrate or $nan {o_3}$ it undergoes the decomposition reaction and results in liberating oxygen $. This reaction takes place at a temperature of. Sodium nitrate undergoes thermal decomposition to produce sodium nitrite and oxygen. On heating sodium nitrate it undergoes decomposition reaction and liberates oxygen & convert into sodium nitrite.