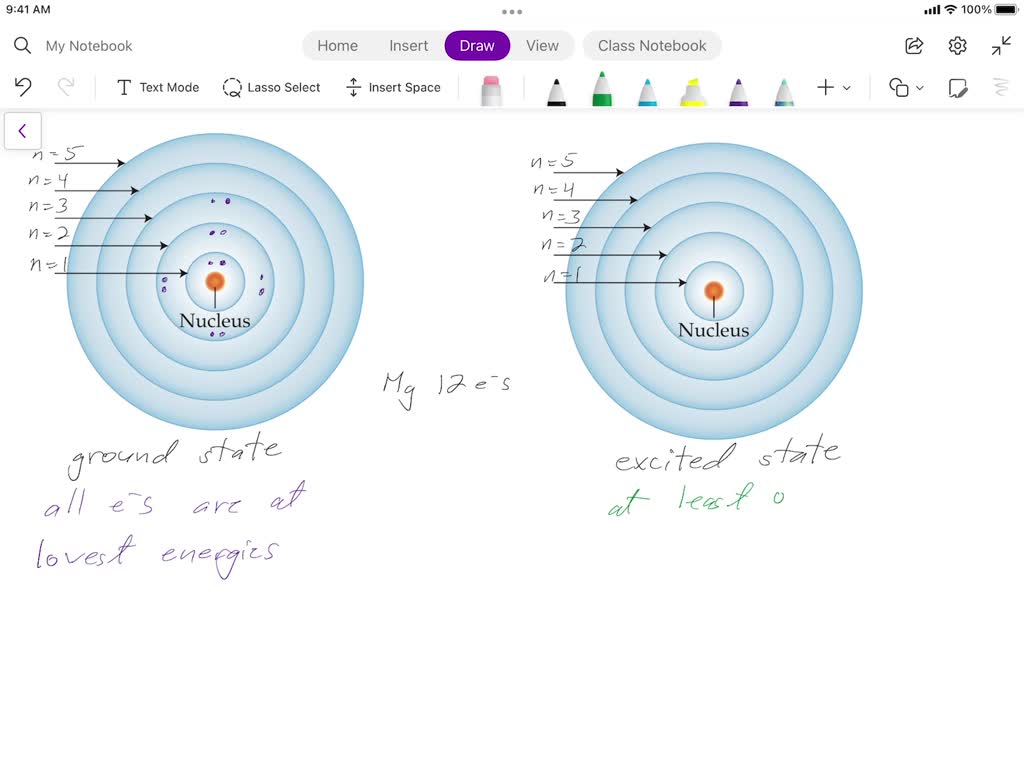
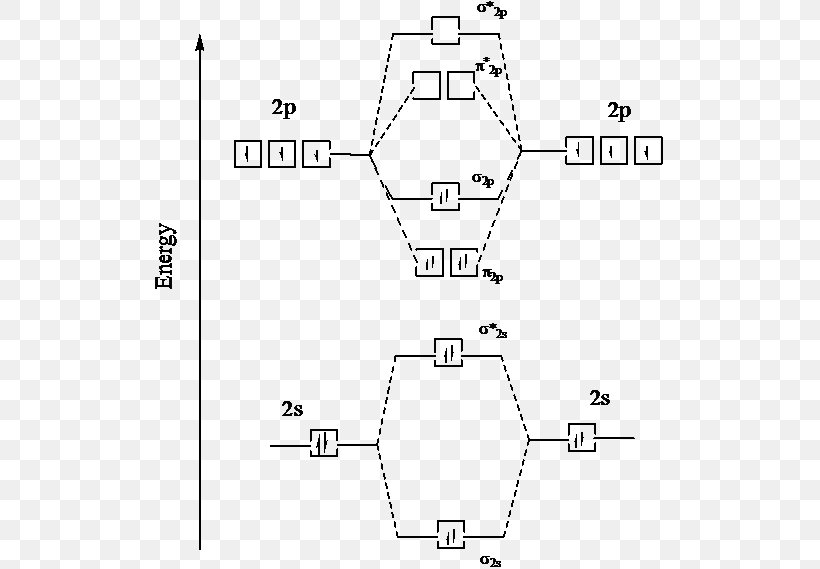
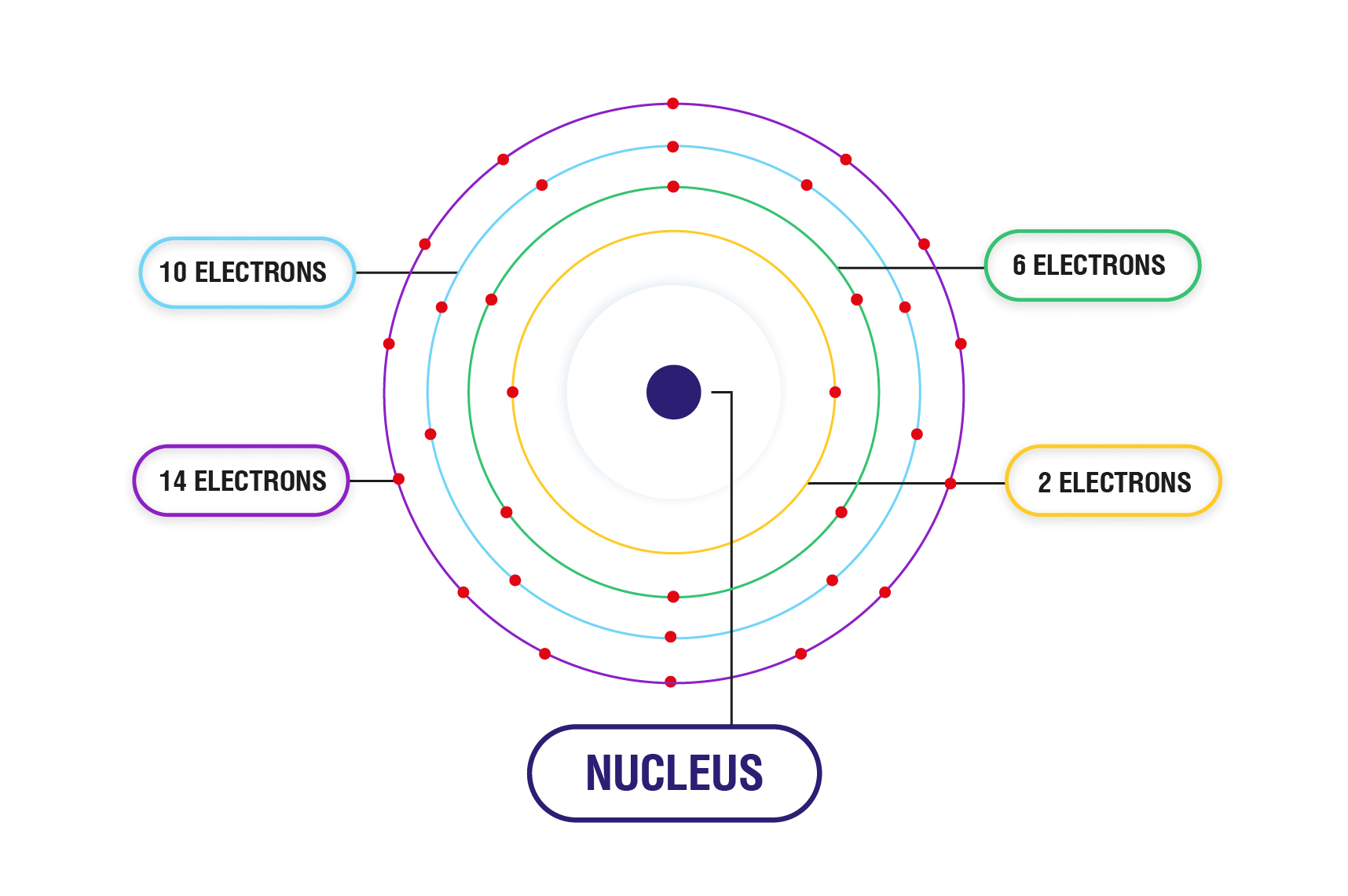
What Is Excited State Electron Configuration
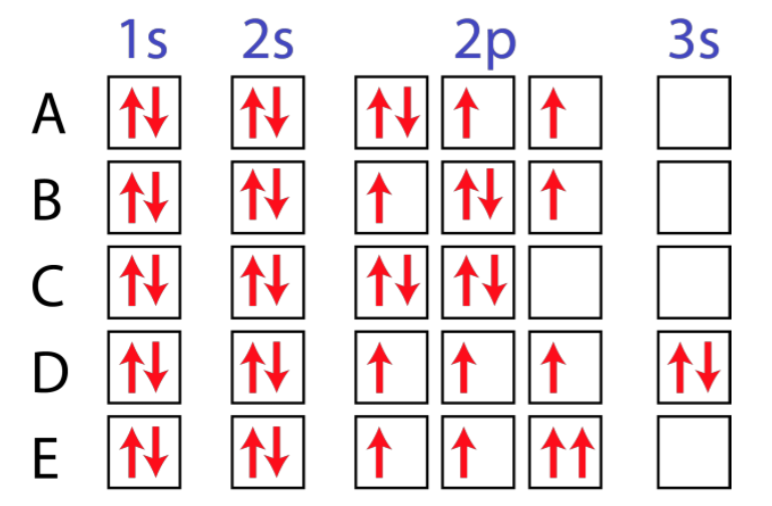
What Is Excited State Electron Configuration - What electron configuration represents an excited state? While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. An excited state means that (typically) the valence electron has. The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the.
An excited state means that (typically) the valence electron has. While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. What electron configuration represents an excited state?
The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the. What electron configuration represents an excited state? While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. An excited state means that (typically) the valence electron has. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state.
65 CR 2+ ELECTRON CONFIGURATION * ElectronConfiguration
The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. What electron configuration.
Solved Which of the following represents an excited state
The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the. An excited state means that (typically) the valence electron has. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a.
😍 Electron configuration examples. Abbreviated Electron configurations
An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the. What electron configuration.
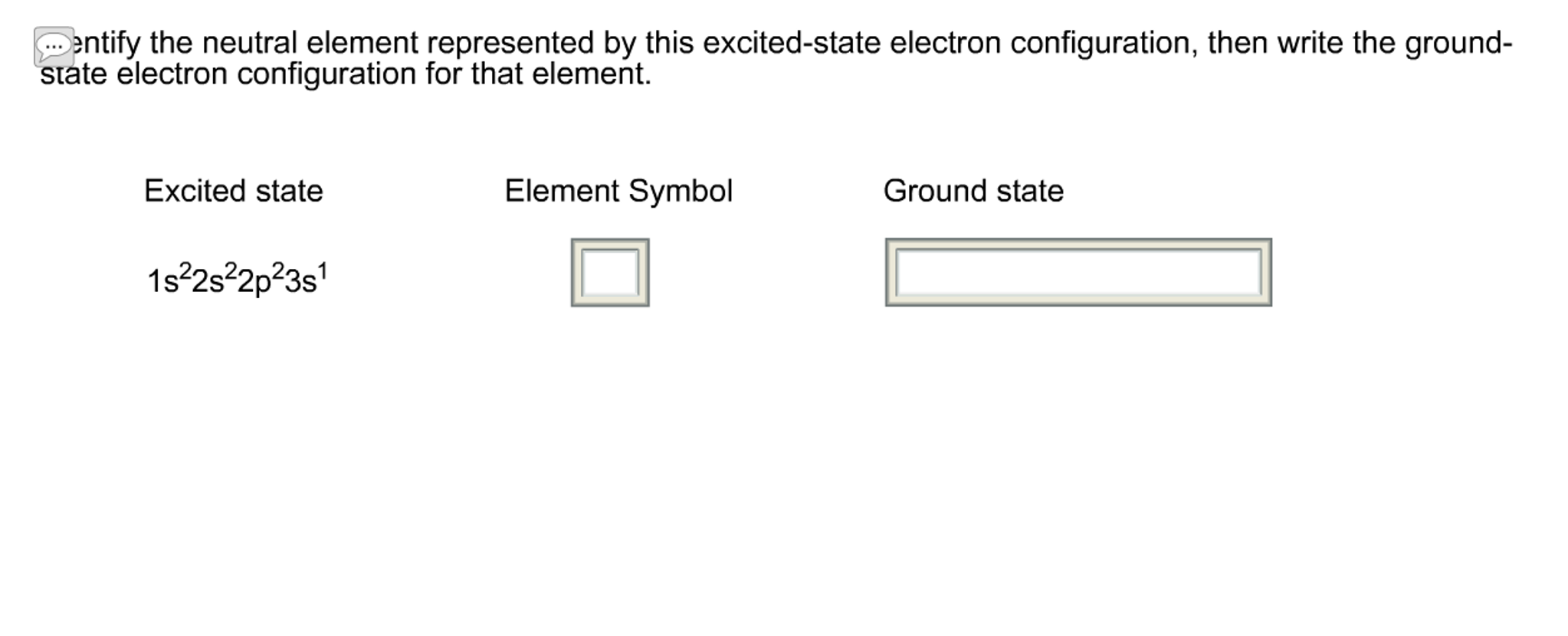
Solved Identify the neutral element represented by this
While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. What electron configuration represents an excited state? An excited state means that (typically) the valence.
10 [TUTORIAL] ELECTRON CONFIGURATION EXCITED STATE with VIDEO + PDF
What electron configuration represents an excited state? While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the. An excited state is an.
SOLVEDWhat is a groundstate electron configuration? What is an
An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. An excited state means that (typically) the valence electron has. The first excited state is.
Electron Configuration Excited State Molecular Orbital Diagram Ground
The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the. What electron configuration represents an excited state? An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level.
Excited State Electron Configuration Calculator
An excited state means that (typically) the valence electron has. While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the. What electron.
Orbital Energy Levels Electron Configurations
An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. An excited state means that (typically) the valence electron has. While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. What electron configuration represents an.
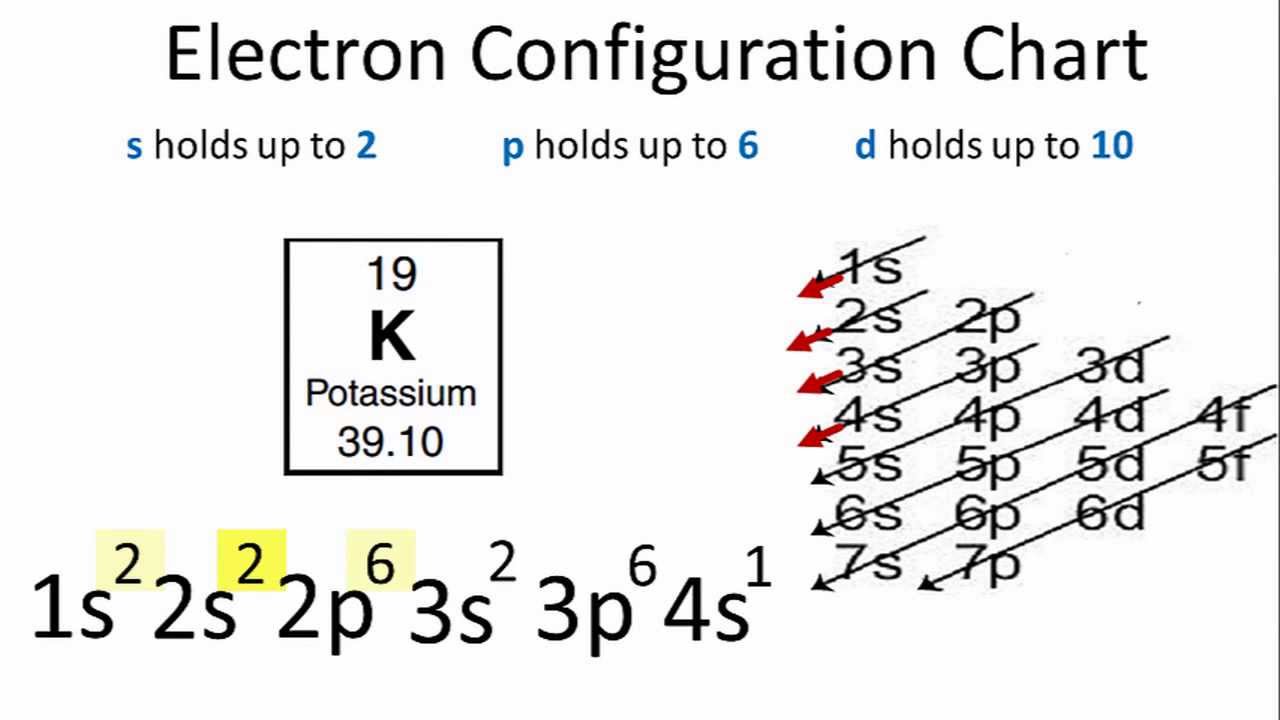
How to Write Ground State Electron Configuration in Chemistry
An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. An excited state means that (typically) the valence electron has. What electron configuration represents an.
An Excited State Means That (Typically) The Valence Electron Has.
What electron configuration represents an excited state? While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in. The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state.
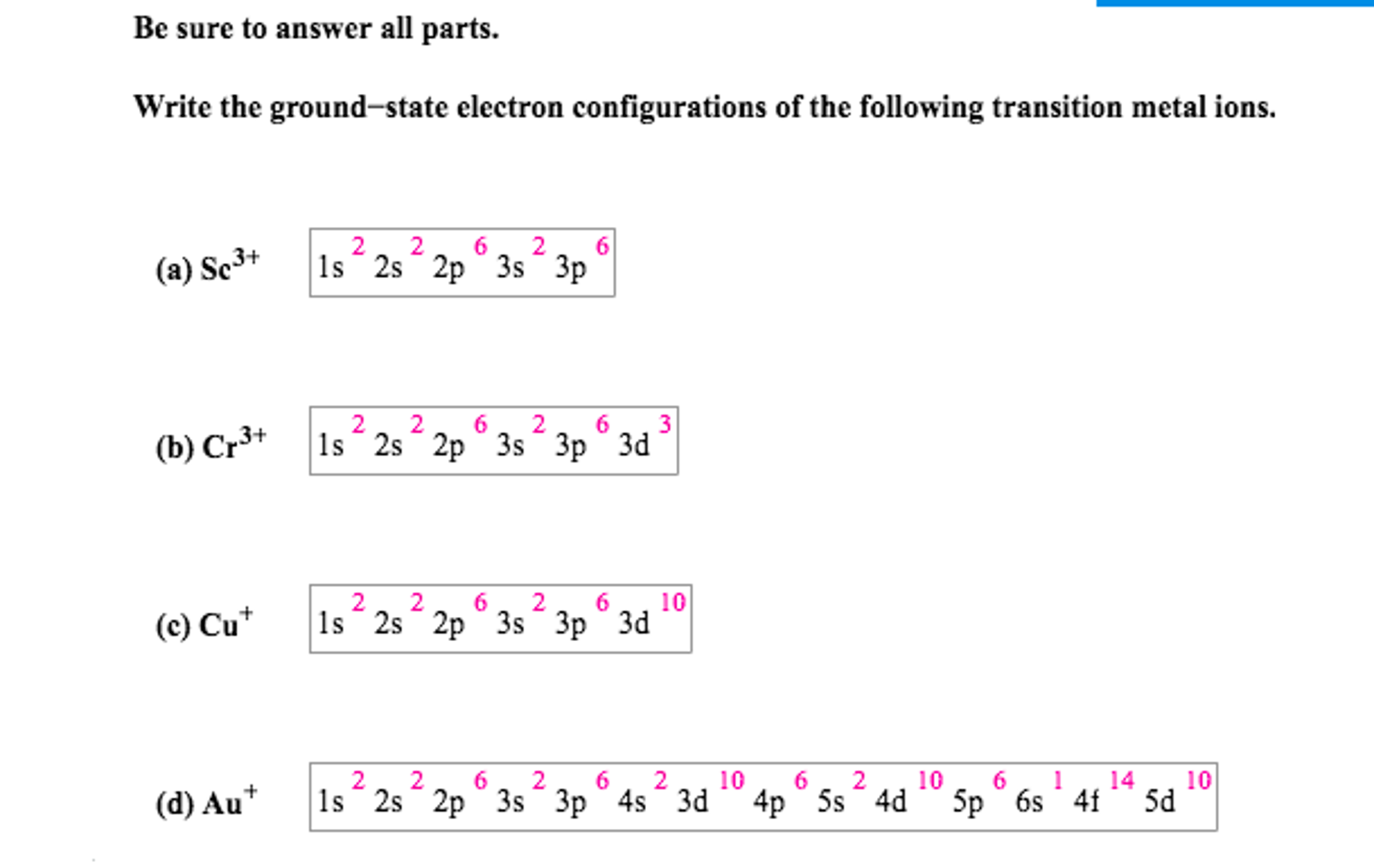
![10 [TUTORIAL] ELECTRON CONFIGURATION EXCITED STATE with VIDEO + PDF](https://image1.slideserve.com/2043186/slide5-n.jpg)