What Is Pseudo Noble Gas Configuration
What Is Pseudo Noble Gas Configuration - A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It is achieved when an atom. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration.
It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons.
It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It is achieved when an atom.
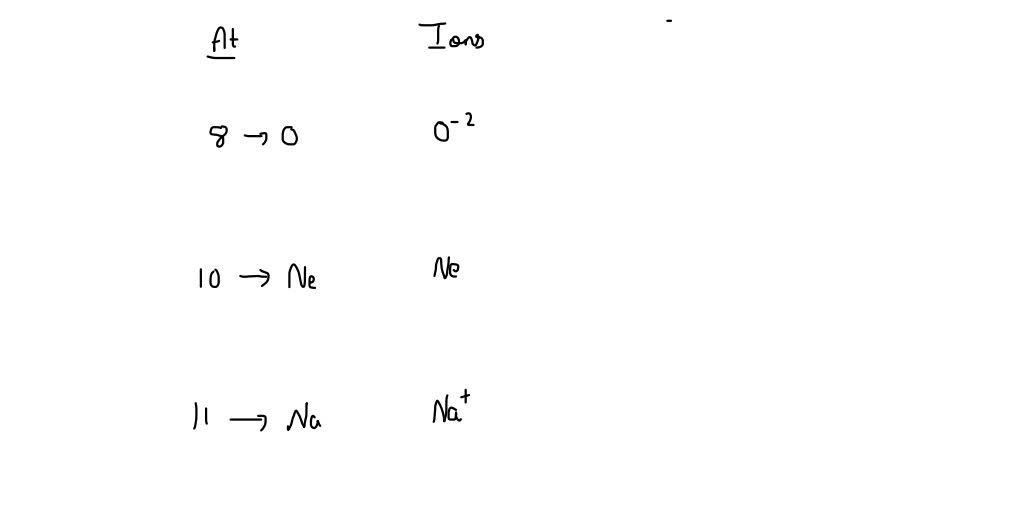
SOLVED Give the Pseudo Noble gas configuration if 8,10,11 ions
A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or.
Noble Gas Configuration For Cobalt Ash in The Wild
A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It refers to elements having 18 electrons instead of just 8 in their outermost.
Noble Gas Configuration Practice Questions
A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. A pseudo noble gas configuration refers to an electron configuration that is similar to.
SECTIONB 86. Pseudo inert gas configuration is Filo
A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two.
Noble Gas Configuration Of Vanadium
It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble.
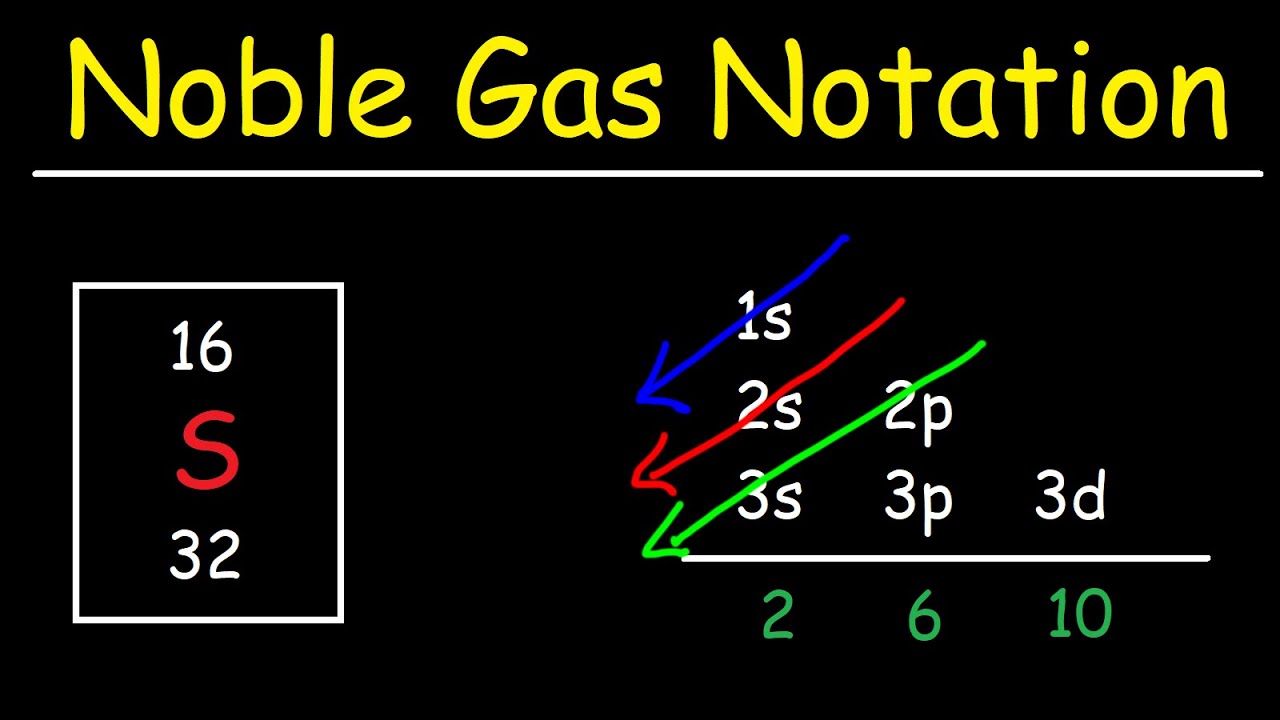
44+ noble gas electron configuration calculator EileenSuzie
A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It is achieved when an atom. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo.
SOLVEDWhat is a pseudonoble gas configuration? Give an example of one
It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It refers to elements having 18 electrons instead of just 8 in.
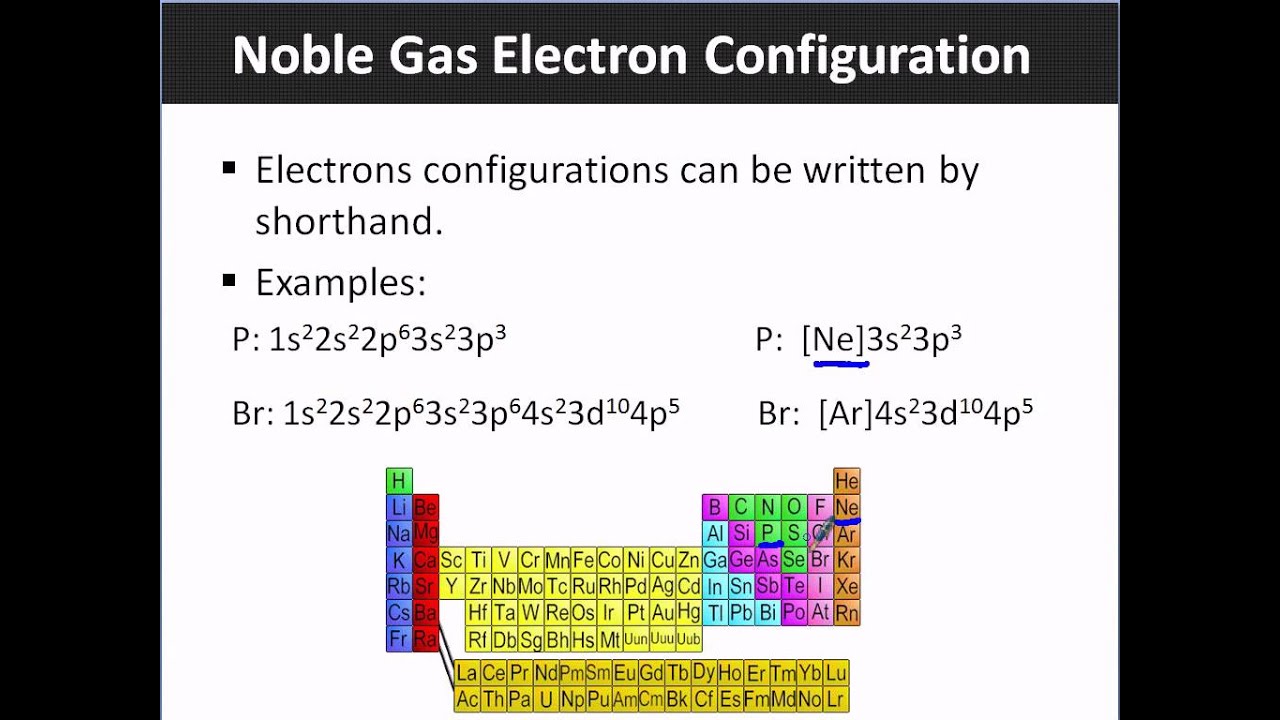
How to Write a Noble Gas Configuration for Atoms of an Element
It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo.
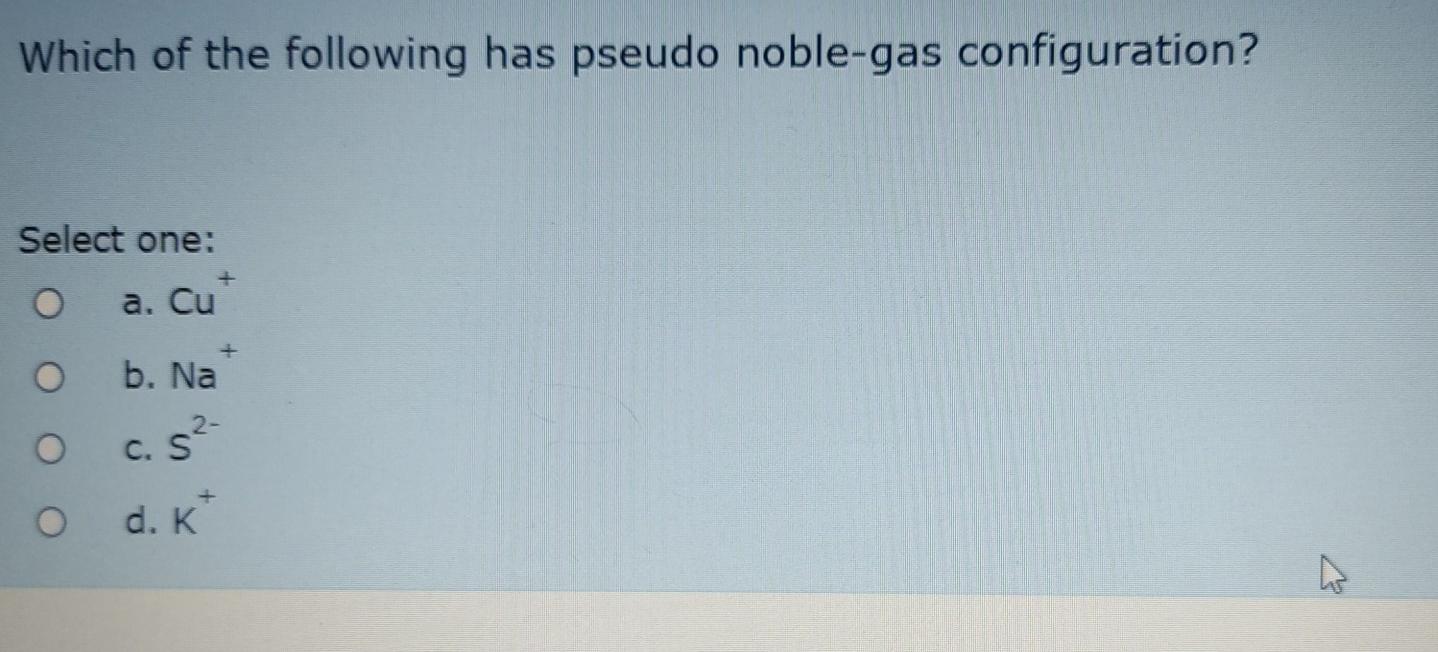
Solved Which of the following has pseudo noblegas
It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It is achieved when an atom. A pseudo noble gas configuration refers to an electron configuration.
Antimony noble gas configuration virtmilitary
It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo.
It Is Achieved When An Atom.
A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons.