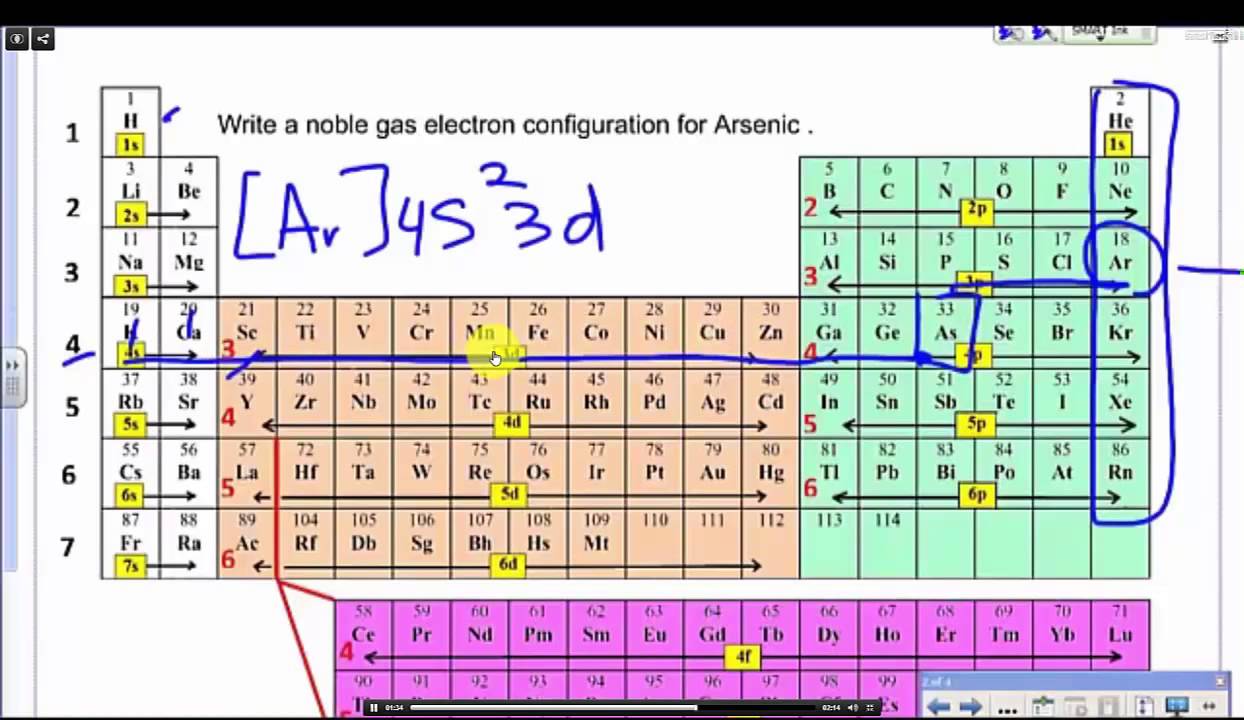
What Is The Abbreviated Electron Configuration Of Mn2
What Is The Abbreviated Electron Configuration Of Mn2 - To determine the abbreviated electron configuration of the m n 2 + ion, start by writing the full electron configuration of a neutral. Electronic configuration of manganese (z = 25) is _____ explain the observation, at the end of each period, there is a slight increase in the atomic. The abbreviated electron configuration of mn is [ar]4s23d5. What is the abbreviated electron configuration of mn2+? The abbreviated electron configuration of mn is [ar]4s23d5. What is the abbreviated electron configuration of mn2+? The electronic configuration of mn is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 2. The electron configuration of mn (manganese) is [ar] 4s2 3d5. An ion has electronic configuration [k r] 4 d 4 placed in group 6 in the modern periodic table. Which of the following is not an ionic compound?
The abbreviated electron configuration of mn is [ar]4s23d5. The abbreviated electron configuration of mn is [ar]4s23d5. The electronic configuration of mn is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 2. To determine the abbreviated electron configuration of the m n 2 + ion, start by writing the full electron configuration of a neutral. An ion has electronic configuration [k r] 4 d 4 placed in group 6 in the modern periodic table. Which of the following is not an ionic compound? When mn loses 2 electrons to form mn2+, it loses the 2 electrons. Electronic configuration of manganese (z = 25) is _____ explain the observation, at the end of each period, there is a slight increase in the atomic. The electron configuration of mn (manganese) is [ar] 4s2 3d5. What is the abbreviated electron configuration of mn2+?
To determine the abbreviated electron configuration of the m n 2 + ion, start by writing the full electron configuration of a neutral. An ion has electronic configuration [k r] 4 d 4 placed in group 6 in the modern periodic table. The electronic configuration of mn is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 2. When mn loses two electrons, then mn 2 + ion is formed. The electron configuration of mn (manganese) is [ar] 4s2 3d5. Electronic configuration of manganese (z = 25) is _____ explain the observation, at the end of each period, there is a slight increase in the atomic. The abbreviated electron configuration of mn is [ar]4s23d5. When mn loses 2 electrons to form mn2+, it loses the 2 electrons. Which of the following is not an ionic compound? What is the abbreviated electron configuration of mn2+?
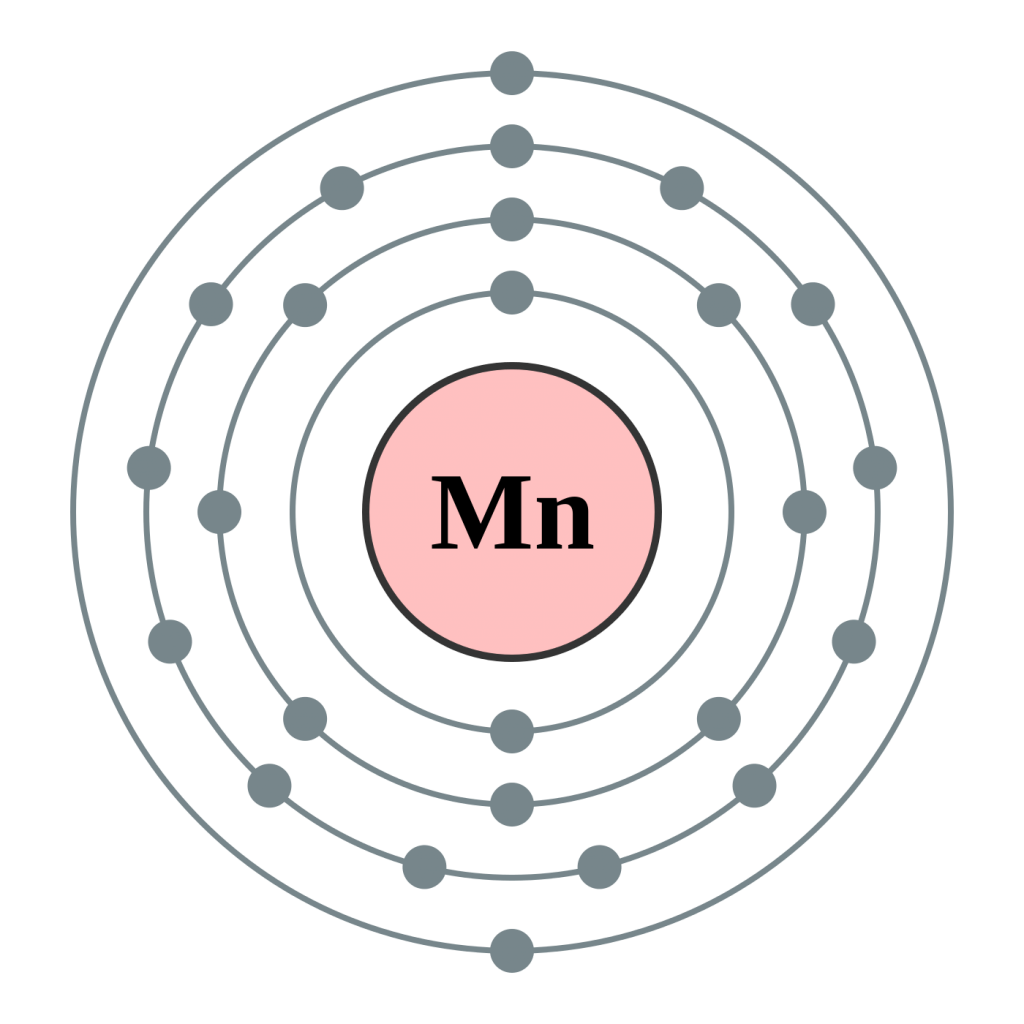
Manganese Electron Configuration (Mn) with Orbital Diagram
The abbreviated electron configuration of mn is [ar]4s23d5. Which of the following is not an ionic compound? The electron configuration of mn (manganese) is [ar] 4s2 3d5. The abbreviated electron configuration of mn is [ar]4s23d5. The electronic configuration of mn is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4.
How To Write Abbreviated Electron
What is the abbreviated electron configuration of mn2+? Electronic configuration of manganese (z = 25) is _____ explain the observation, at the end of each period, there is a slight increase in the atomic. When mn loses two electrons, then mn 2 + ion is formed. When mn loses 2 electrons to form mn2+, it loses the 2 electrons. The.
How To Write Abbreviated Electron
An ion has electronic configuration [k r] 4 d 4 placed in group 6 in the modern periodic table. What is the abbreviated electron configuration of mn2+? Electronic configuration of manganese (z = 25) is _____ explain the observation, at the end of each period, there is a slight increase in the atomic. What is the abbreviated electron configuration of.
😍 Electron configuration examples. Abbreviated Electron configurations
What is the abbreviated electron configuration of mn2+? Electronic configuration of manganese (z = 25) is _____ explain the observation, at the end of each period, there is a slight increase in the atomic. When mn loses two electrons, then mn 2 + ion is formed. To determine the abbreviated electron configuration of the m n 2 + ion, start.
Solved Enter the abbreviated electron configuration for
An ion has electronic configuration [k r] 4 d 4 placed in group 6 in the modern periodic table. When mn loses two electrons, then mn 2 + ion is formed. Which of the following is not an ionic compound? To determine the abbreviated electron configuration of the m n 2 + ion, start by writing the full electron configuration.
Electron configuration PPT
The abbreviated electron configuration of mn is [ar]4s23d5. Electronic configuration of manganese (z = 25) is _____ explain the observation, at the end of each period, there is a slight increase in the atomic. The abbreviated electron configuration of mn is [ar]4s23d5. The electron configuration of mn (manganese) is [ar] 4s2 3d5. When mn loses two electrons, then mn 2.
Abbreviated electron configuration calculator [Noble gas]
When mn loses 2 electrons to form mn2+, it loses the 2 electrons. What is the abbreviated electron configuration of mn2+? Which of the following is not an ionic compound? What is the abbreviated electron configuration of mn2+? The electronic configuration of mn is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3.
How To Write Abbreviated Electron
Electronic configuration of manganese (z = 25) is _____ explain the observation, at the end of each period, there is a slight increase in the atomic. What is the abbreviated electron configuration of mn2+? What is the abbreviated electron configuration of mn2+? The abbreviated electron configuration of mn is [ar]4s23d5. When mn loses 2 electrons to form mn2+, it loses.
65 CR 2+ ELECTRON CONFIGURATION * ElectronConfiguration
To determine the abbreviated electron configuration of the m n 2 + ion, start by writing the full electron configuration of a neutral. Electronic configuration of manganese (z = 25) is _____ explain the observation, at the end of each period, there is a slight increase in the atomic. An ion has electronic configuration [k r] 4 d 4 placed.
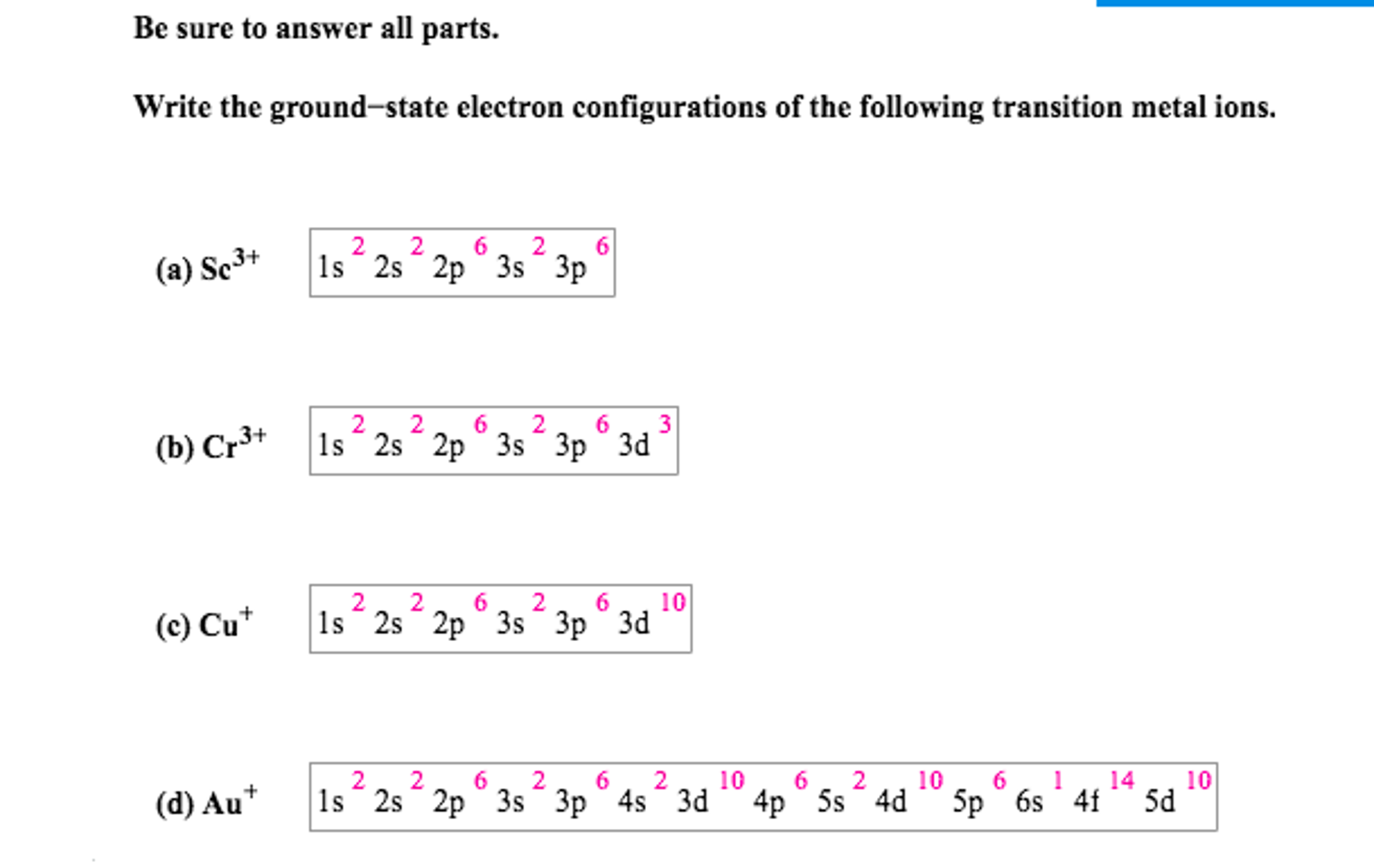
[Solved] Write abbreviated ground state electron configuration of each
The abbreviated electron configuration of mn is [ar]4s23d5. What is the abbreviated electron configuration of mn2+? An ion has electronic configuration [k r] 4 d 4 placed in group 6 in the modern periodic table. The electron configuration of mn (manganese) is [ar] 4s2 3d5. Which of the following is not an ionic compound?
What Is The Abbreviated Electron Configuration Of Mn2+?
The abbreviated electron configuration of mn is [ar]4s23d5. What is the abbreviated electron configuration of mn2+? An ion has electronic configuration [k r] 4 d 4 placed in group 6 in the modern periodic table. Which of the following is not an ionic compound?
The Electron Configuration Of Mn (Manganese) Is [Ar] 4S2 3D5.
The abbreviated electron configuration of mn is [ar]4s23d5. To determine the abbreviated electron configuration of the m n 2 + ion, start by writing the full electron configuration of a neutral. When mn loses two electrons, then mn 2 + ion is formed. The electronic configuration of mn is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 2.
Electronic Configuration Of Manganese (Z = 25) Is _____ Explain The Observation, At The End Of Each Period, There Is A Slight Increase In The Atomic.
When mn loses 2 electrons to form mn2+, it loses the 2 electrons.
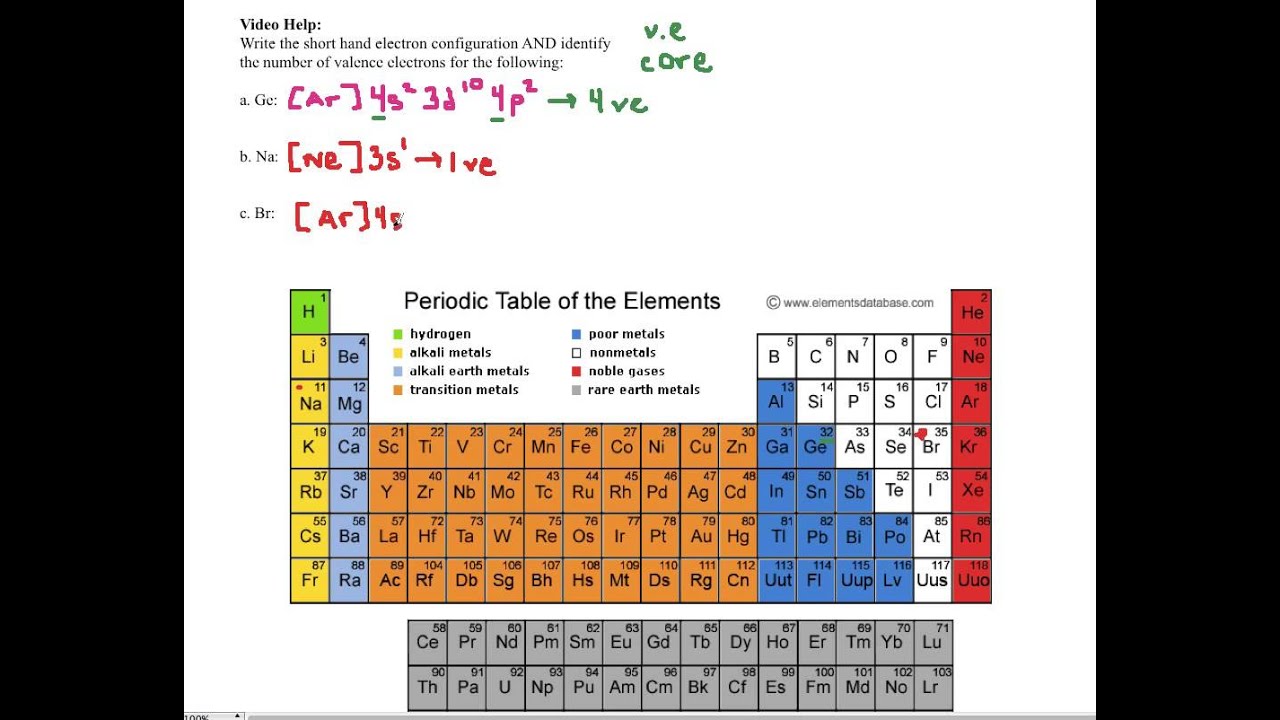
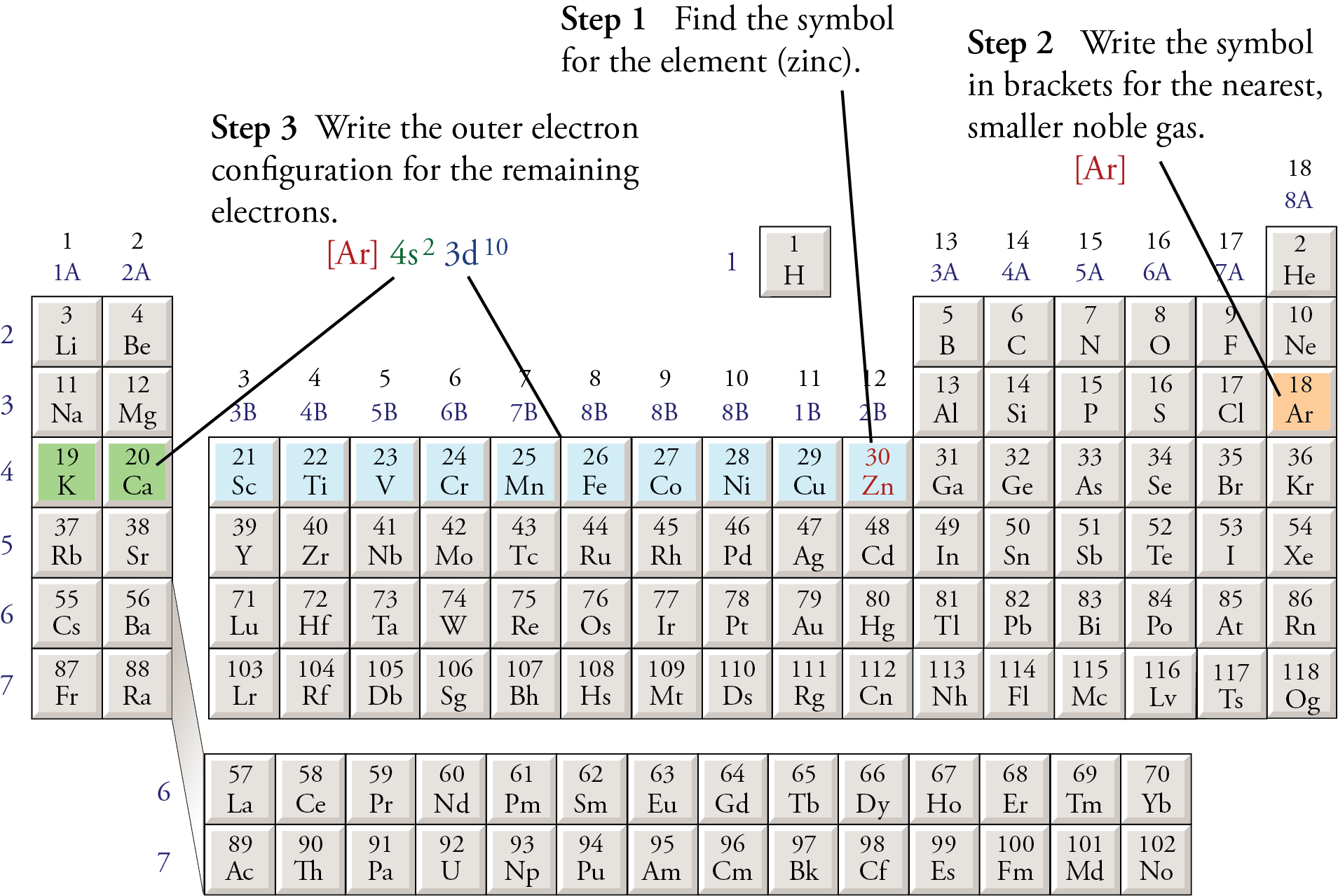
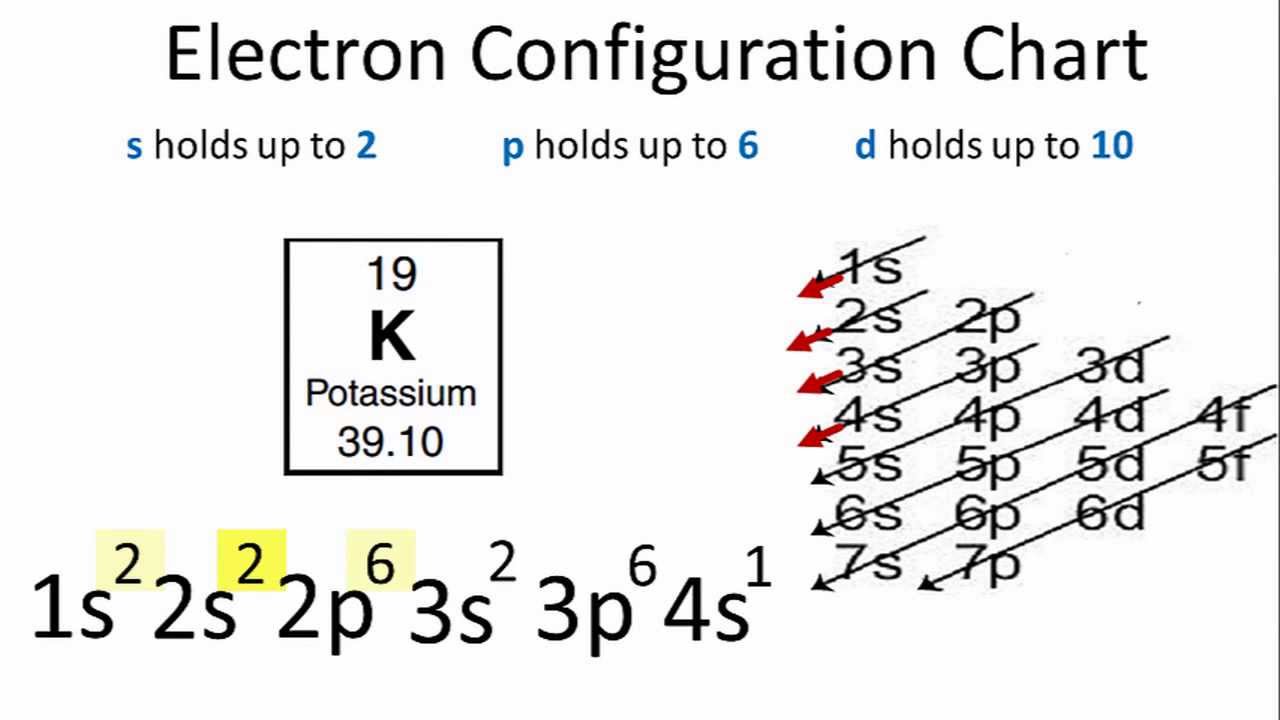
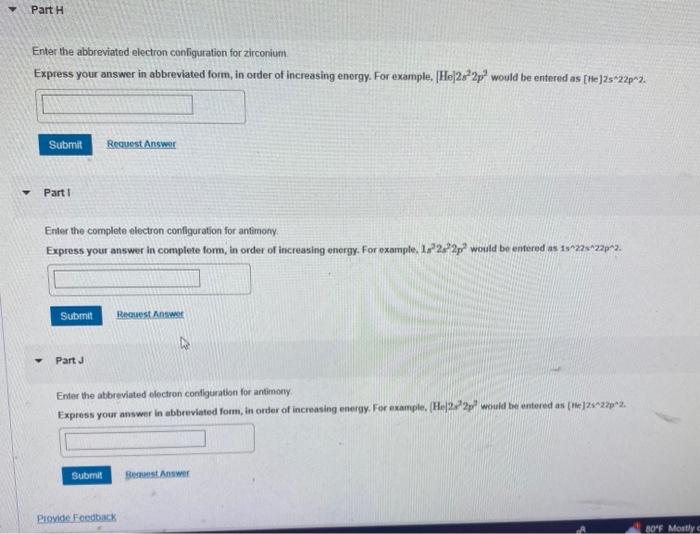

![Abbreviated electron configuration calculator [Noble gas]](https://topblogtenz.com/wp-content/uploads/2022/04/how-to-write-shorthand-or-noble-gas-electron-configuration-min.png)