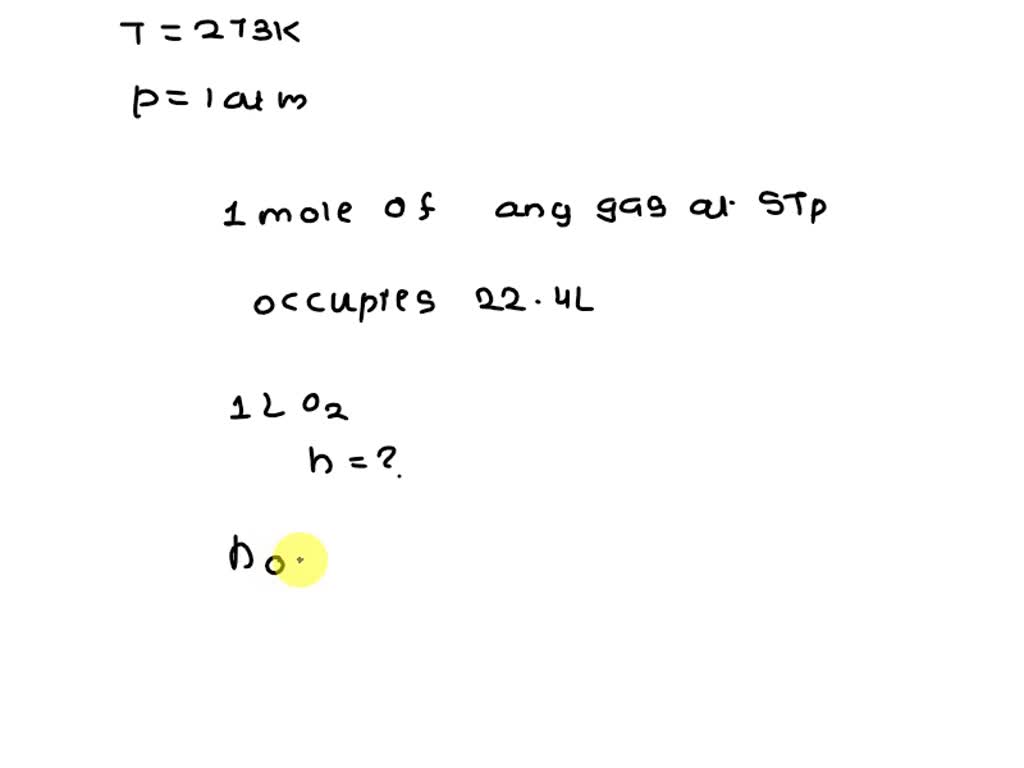
What Is The Average Distance Between Oxygen Molecules At Stp
What Is The Average Distance Between Oxygen Molecules At Stp - There are 3 steps to solve this one. Stp is defined as a temperature. Try putting each molecule in a cube, so that the volume of each cube is determined by the idea gas law, and the average distance between. The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp is very close to zero. Finally, we can find the average distance between oxygen molecules by dividing the length of one side of the cube by the. Stp refers to standard temperature pressure, this is used to ensure the standardization of the envir. The distance between an atom's nucleus. First, we need to find the volume occupied by one mole of oxygen gas at stp (standard temperature and pressure).
There are 3 steps to solve this one. Finally, we can find the average distance between oxygen molecules by dividing the length of one side of the cube by the. Stp is defined as a temperature. Try putting each molecule in a cube, so that the volume of each cube is determined by the idea gas law, and the average distance between. The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp is very close to zero. Stp refers to standard temperature pressure, this is used to ensure the standardization of the envir. The distance between an atom's nucleus. First, we need to find the volume occupied by one mole of oxygen gas at stp (standard temperature and pressure).
Stp is defined as a temperature. There are 3 steps to solve this one. The distance between an atom's nucleus. Stp refers to standard temperature pressure, this is used to ensure the standardization of the envir. The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp is very close to zero. First, we need to find the volume occupied by one mole of oxygen gas at stp (standard temperature and pressure). Finally, we can find the average distance between oxygen molecules by dividing the length of one side of the cube by the. Try putting each molecule in a cube, so that the volume of each cube is determined by the idea gas law, and the average distance between.
SOLVED(II) What is the average distance between nitrogen molecules at STP?
The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp is very close to zero. Finally, we can find the average distance between oxygen molecules by dividing the length of one side of the cube by the. First, we need to find the volume occupied by one mole of oxygen gas at.
SOLVED What is the average distance between air molecules at STP
There are 3 steps to solve this one. Stp refers to standard temperature pressure, this is used to ensure the standardization of the envir. Stp is defined as a temperature. Finally, we can find the average distance between oxygen molecules by dividing the length of one side of the cube by the. First, we need to find the volume occupied.
SOLVED The number of molecules in a litre of oxygen at STP is
Try putting each molecule in a cube, so that the volume of each cube is determined by the idea gas law, and the average distance between. Stp is defined as a temperature. Stp refers to standard temperature pressure, this is used to ensure the standardization of the envir. Finally, we can find the average distance between oxygen molecules by dividing.
SOLVED(II) What is the average distance between oxygen molecules at STP?
There are 3 steps to solve this one. Try putting each molecule in a cube, so that the volume of each cube is determined by the idea gas law, and the average distance between. The distance between an atom's nucleus. First, we need to find the volume occupied by one mole of oxygen gas at stp (standard temperature and pressure)..
SOLVEDOne breath of air has a volume of 2 L at STP. If air contains 20
The distance between an atom's nucleus. The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp is very close to zero. Try putting each molecule in a cube, so that the volume of each cube is determined by the idea gas law, and the average distance between. Stp refers to standard temperature.
Calculate the r.m.s. Velocity of Oxygen molecules STP the molecular
Stp is defined as a temperature. The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp is very close to zero. There are 3 steps to solve this one. Finally, we can find the average distance between oxygen molecules by dividing the length of one side of the cube by the. Try.
sity STP. Calculate the rms speed of the gas molecules. 14. The average
Finally, we can find the average distance between oxygen molecules by dividing the length of one side of the cube by the. The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp is very close to zero. First, we need to find the volume occupied by one mole of oxygen gas at.
SOLVED Calculate the volume at stp occupied by 10 21 molecules of oxygen
The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp is very close to zero. Stp is defined as a temperature. First, we need to find the volume occupied by one mole of oxygen gas at stp (standard temperature and pressure). The distance between an atom's nucleus. There are 3 steps to.
SOLVEDWhat is the average distance between nitrogen molecules at STP?
The distance between an atom's nucleus. Stp refers to standard temperature pressure, this is used to ensure the standardization of the envir. First, we need to find the volume occupied by one mole of oxygen gas at stp (standard temperature and pressure). The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp.
Q. If the average speed of O2 molecules at STP is 4.3×102 m/s. What is t..
There are 3 steps to solve this one. First, we need to find the volume occupied by one mole of oxygen gas at stp (standard temperature and pressure). Stp is defined as a temperature. Finally, we can find the average distance between oxygen molecules by dividing the length of one side of the cube by the. The distance between an.
Finally, We Can Find The Average Distance Between Oxygen Molecules By Dividing The Length Of One Side Of The Cube By The.
Stp is defined as a temperature. The volume of one oxygen molecule is incredibly small, so the average distance between oxygen molecules at stp is very close to zero. First, we need to find the volume occupied by one mole of oxygen gas at stp (standard temperature and pressure). Try putting each molecule in a cube, so that the volume of each cube is determined by the idea gas law, and the average distance between.
Stp Refers To Standard Temperature Pressure, This Is Used To Ensure The Standardization Of The Envir.
There are 3 steps to solve this one. The distance between an atom's nucleus.