What Is The Bond Order Of Be2
What Is The Bond Order Of Be2 - The total number of electrons in be2 is 4 (2 from each beryllium. The bond order of be2− is 0 since it has an equal number of bonding and antibonding electrons, resulting in an unstable molecule. First, the electronic configuration of the beryllium atom in its gro. To calculate the bond order of the molecule be2, we can follow these steps: Not the question you’re looking for?. There are 2 steps to solve this one. If one of the electrons in the b e 2 molecule is taken to the next excited state, then the bond order in b e 2 is:
There are 2 steps to solve this one. First, the electronic configuration of the beryllium atom in its gro. The total number of electrons in be2 is 4 (2 from each beryllium. If one of the electrons in the b e 2 molecule is taken to the next excited state, then the bond order in b e 2 is: The bond order of be2− is 0 since it has an equal number of bonding and antibonding electrons, resulting in an unstable molecule. Not the question you’re looking for?. To calculate the bond order of the molecule be2, we can follow these steps:
First, the electronic configuration of the beryllium atom in its gro. If one of the electrons in the b e 2 molecule is taken to the next excited state, then the bond order in b e 2 is: There are 2 steps to solve this one. The total number of electrons in be2 is 4 (2 from each beryllium. To calculate the bond order of the molecule be2, we can follow these steps: The bond order of be2− is 0 since it has an equal number of bonding and antibonding electrons, resulting in an unstable molecule. Not the question you’re looking for?.
SOLVED What is the bond order of Bez Express the bond order
If one of the electrons in the b e 2 molecule is taken to the next excited state, then the bond order in b e 2 is: There are 2 steps to solve this one. The total number of electrons in be2 is 4 (2 from each beryllium. The bond order of be2− is 0 since it has an equal.
31+ calculate the bond order of b2 MarionCorrin
There are 2 steps to solve this one. To calculate the bond order of the molecule be2, we can follow these steps: Not the question you’re looking for?. The total number of electrons in be2 is 4 (2 from each beryllium. If one of the electrons in the b e 2 molecule is taken to the next excited state, then.
Be2 Molecular Orbital Diagram Bond Order
The bond order of be2− is 0 since it has an equal number of bonding and antibonding electrons, resulting in an unstable molecule. First, the electronic configuration of the beryllium atom in its gro. If one of the electrons in the b e 2 molecule is taken to the next excited state, then the bond order in b e 2.
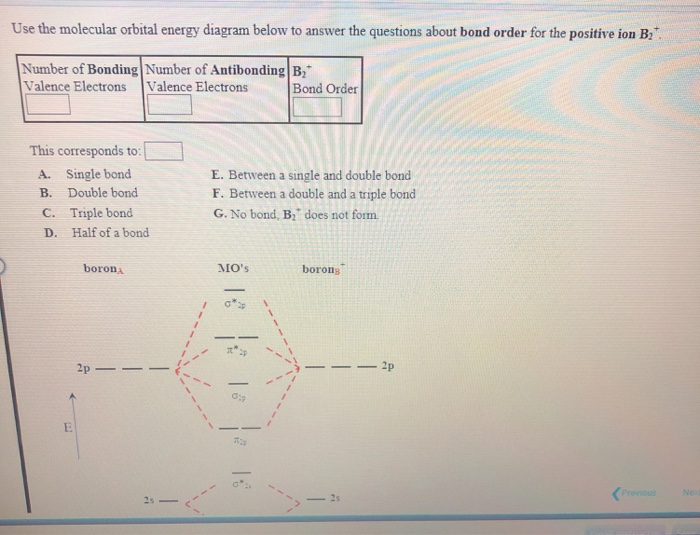
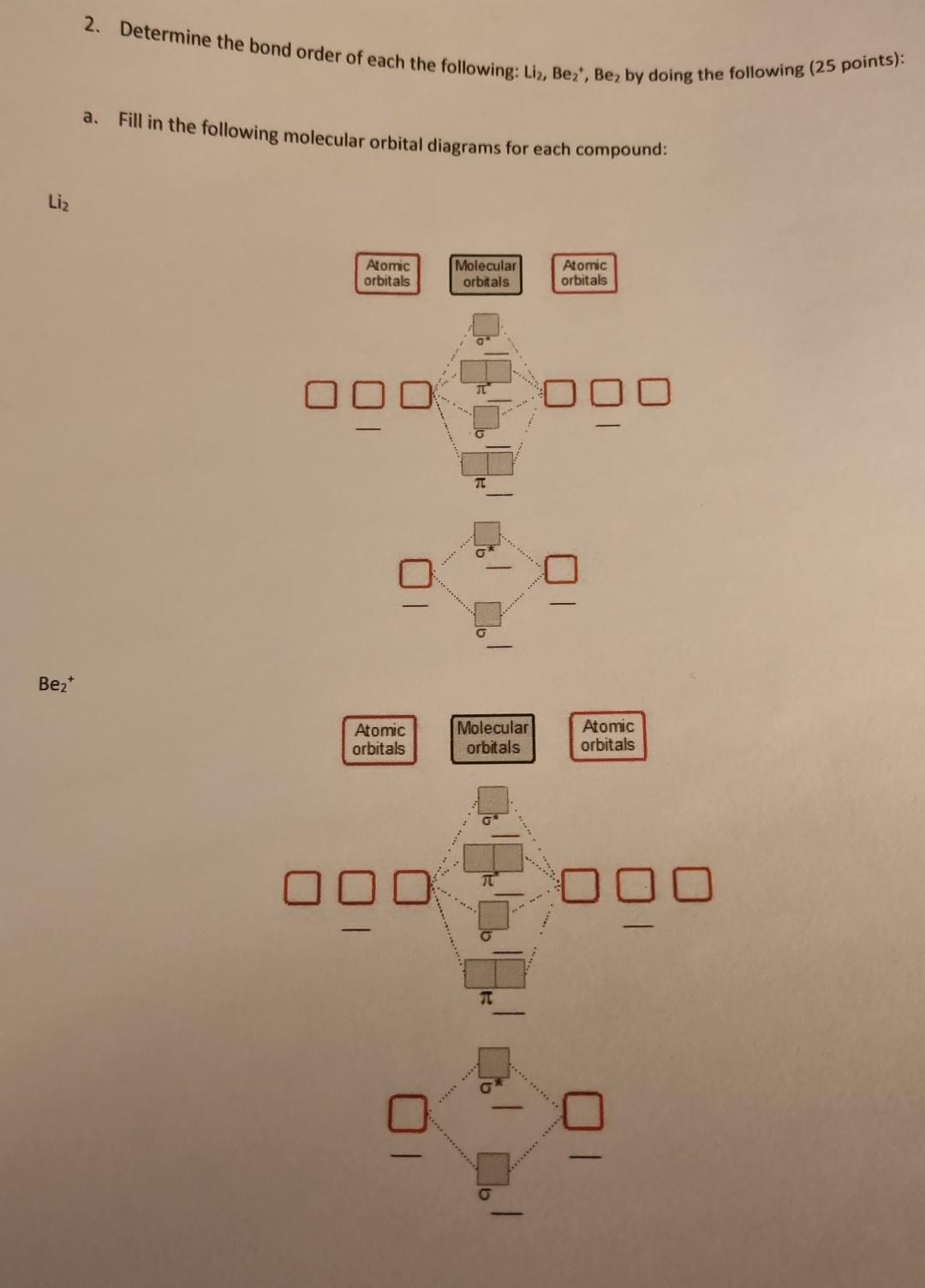
Solved 2. Determine the bond order nf an.L.. pints)b.
First, the electronic configuration of the beryllium atom in its gro. If one of the electrons in the b e 2 molecule is taken to the next excited state, then the bond order in b e 2 is: The bond order of be2− is 0 since it has an equal number of bonding and antibonding electrons, resulting in an unstable.
Solved 2. Determine the bond order nf an.L.. pints)b.
The bond order of be2− is 0 since it has an equal number of bonding and antibonding electrons, resulting in an unstable molecule. There are 2 steps to solve this one. To calculate the bond order of the molecule be2, we can follow these steps: The total number of electrons in be2 is 4 (2 from each beryllium. If one.
(a) What are the relationships among bond order, bond length, and bond
If one of the electrons in the b e 2 molecule is taken to the next excited state, then the bond order in b e 2 is: There are 2 steps to solve this one. The total number of electrons in be2 is 4 (2 from each beryllium. First, the electronic configuration of the beryllium atom in its gro. The.
Find out the bond order ofBe2
The total number of electrons in be2 is 4 (2 from each beryllium. To calculate the bond order of the molecule be2, we can follow these steps: The bond order of be2− is 0 since it has an equal number of bonding and antibonding electrons, resulting in an unstable molecule. There are 2 steps to solve this one. If one.
Solved Part A What is the bond order of Be2? Express the
Not the question you’re looking for?. There are 2 steps to solve this one. If one of the electrons in the b e 2 molecule is taken to the next excited state, then the bond order in b e 2 is: The total number of electrons in be2 is 4 (2 from each beryllium. To calculate the bond order of.
Solved Part A What is the bond order of Be2? Express the
To calculate the bond order of the molecule be2, we can follow these steps: The bond order of be2− is 0 since it has an equal number of bonding and antibonding electrons, resulting in an unstable molecule. The total number of electrons in be2 is 4 (2 from each beryllium. Not the question you’re looking for?. First, the electronic configuration.
Solved 2. Determine the bond order nf an.L.. pints)b.
Not the question you’re looking for?. First, the electronic configuration of the beryllium atom in its gro. The bond order of be2− is 0 since it has an equal number of bonding and antibonding electrons, resulting in an unstable molecule. There are 2 steps to solve this one. To calculate the bond order of the molecule be2, we can follow.
Not The Question You’re Looking For?.
To calculate the bond order of the molecule be2, we can follow these steps: There are 2 steps to solve this one. If one of the electrons in the b e 2 molecule is taken to the next excited state, then the bond order in b e 2 is: First, the electronic configuration of the beryllium atom in its gro.
The Bond Order Of Be2− Is 0 Since It Has An Equal Number Of Bonding And Antibonding Electrons, Resulting In An Unstable Molecule.
The total number of electrons in be2 is 4 (2 from each beryllium.