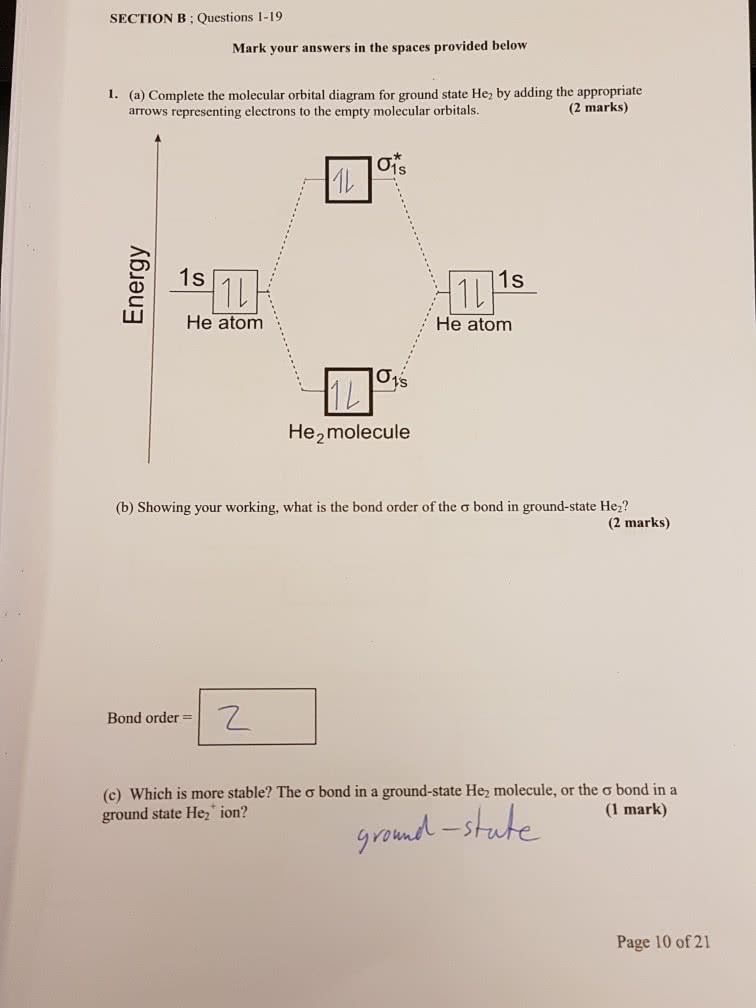
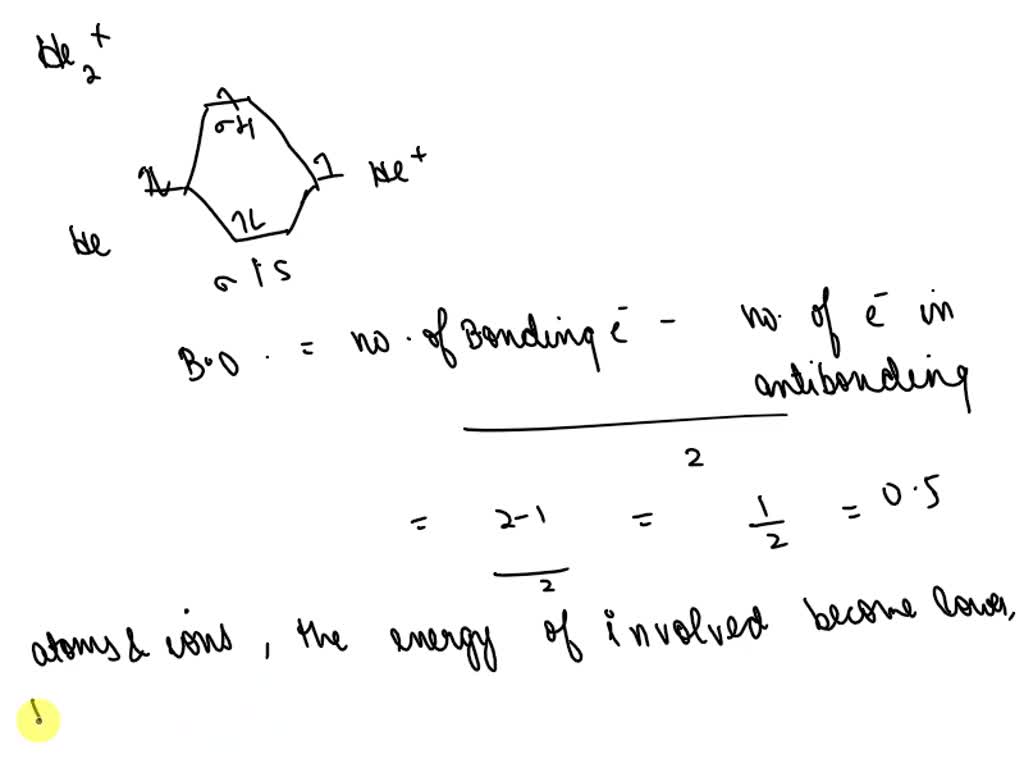What Is The Bond Order Of He2
What Is The Bond Order Of He2 - We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. Bond order is 0.5, and. [2] this chemical is the largest diatomic. Here’s the best way to solve it. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. This means that the bond between the two. The he2+ ion is formed by removing two. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by.
Here’s the best way to solve it. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. [2] this chemical is the largest diatomic. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. The he2+ ion is formed by removing two. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. Bond order is 0.5, and. This means that the bond between the two. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms.
Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. The he2+ ion is formed by removing two. Bond order is 0.5, and. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. This means that the bond between the two. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. [2] this chemical is the largest diatomic. Here’s the best way to solve it. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions.
OneClass The bond order of a He2+ ion is a. 0 b. 0.5 c. 1 d. 1.5 e. 2
The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. Bond order is 0.5, and. The he2+ ion is formed by removing two. We can determine the bond order of a molecule by subtracting.
What is the bond order of the He2 molecule Answer as fast as you can
[2] this chemical is the largest diatomic. The he2+ ion is formed by removing two. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by..
SOLVED What is the bond order of the He2+ ion? Would you expect this
The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. The he2+ ion.
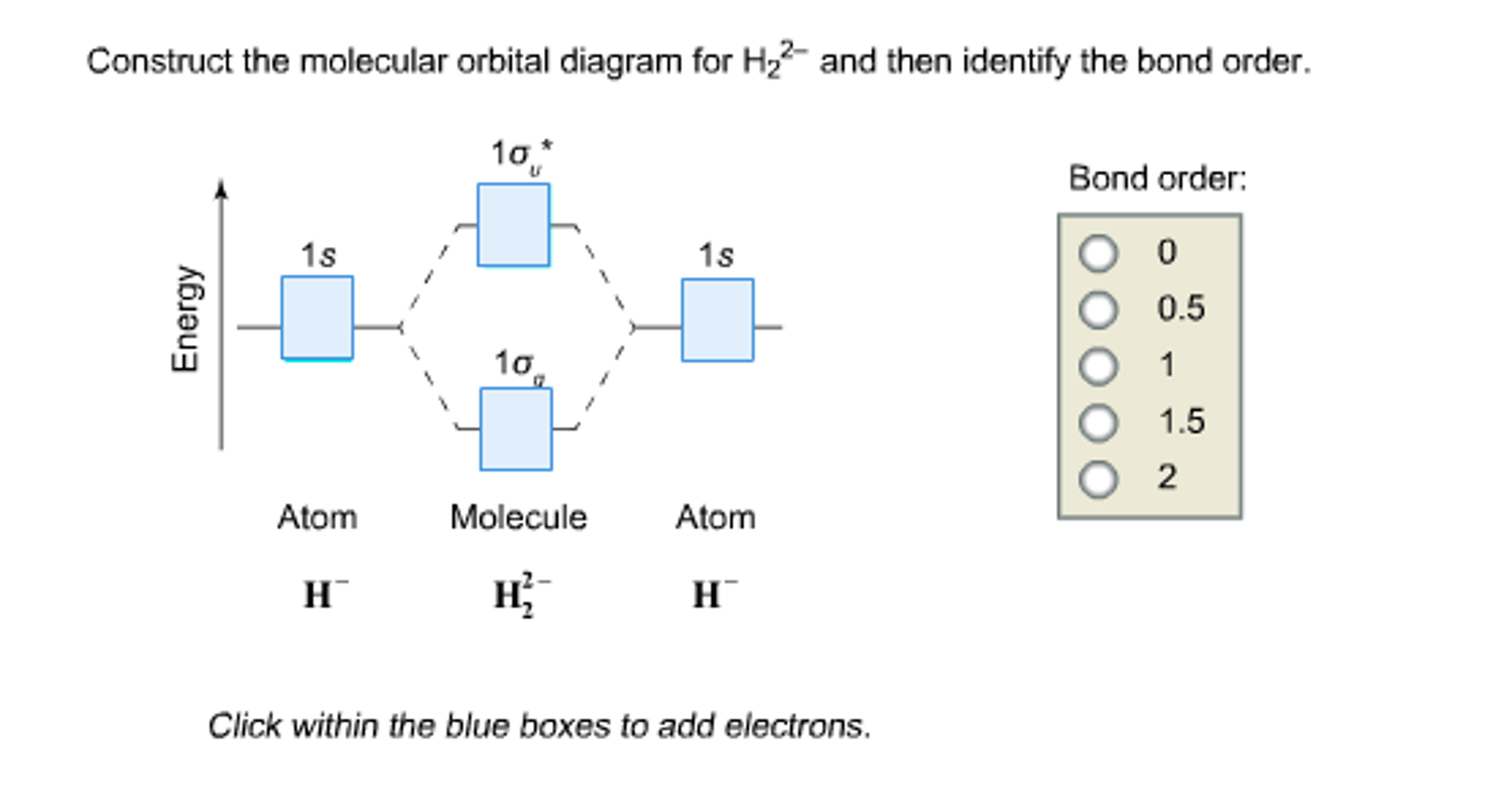
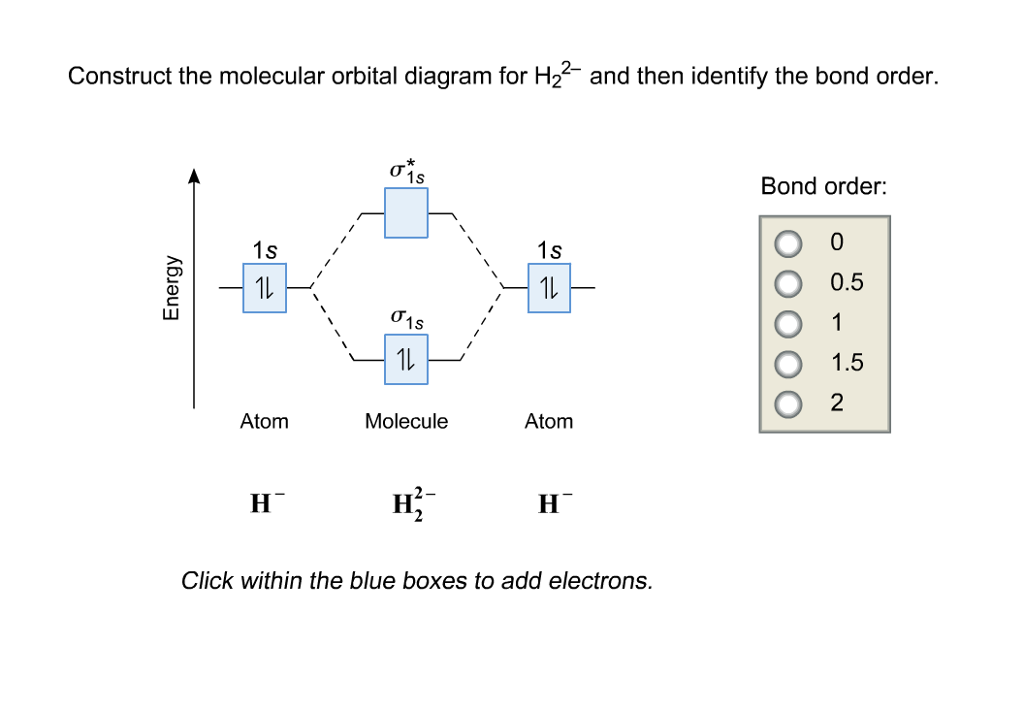
Solved Construct the molecular orbital diagram for H_2^2
To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. [2] this chemical is the largest diatomic. The he2+ ion is formed by removing two. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. We can determine the bond order.
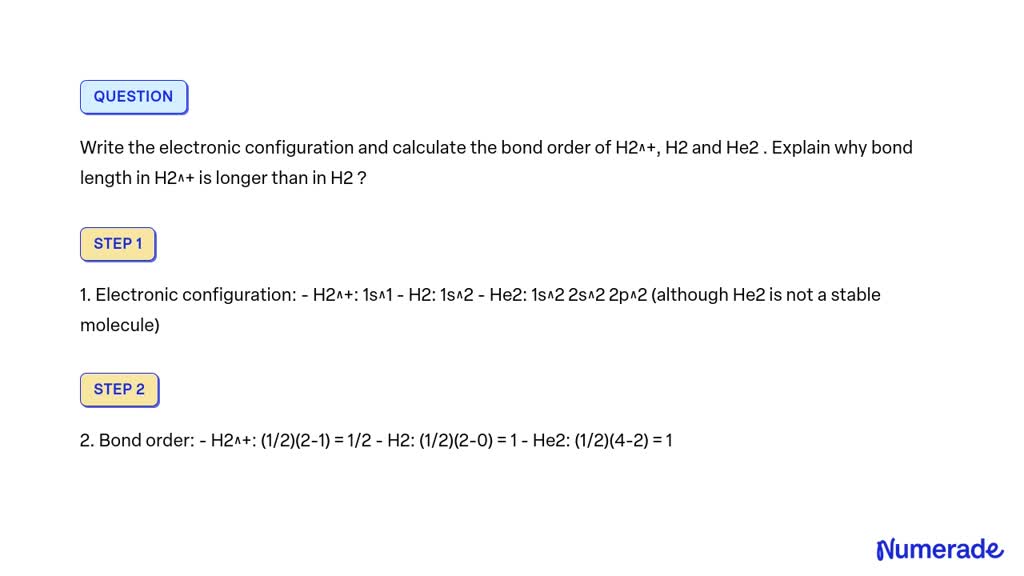
SOLVED Write the electronic configuration and calculate the bond order
The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. [2] this chemical is the largest diatomic. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. To find the bond order of h e 2 +, first determine the number of bonding electrons.
What is Bond order of H2? UO Chemists
Bond order is 0.5, and. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. Here’s the best way to solve it. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. Draw an mo diagram to predict the bond order.
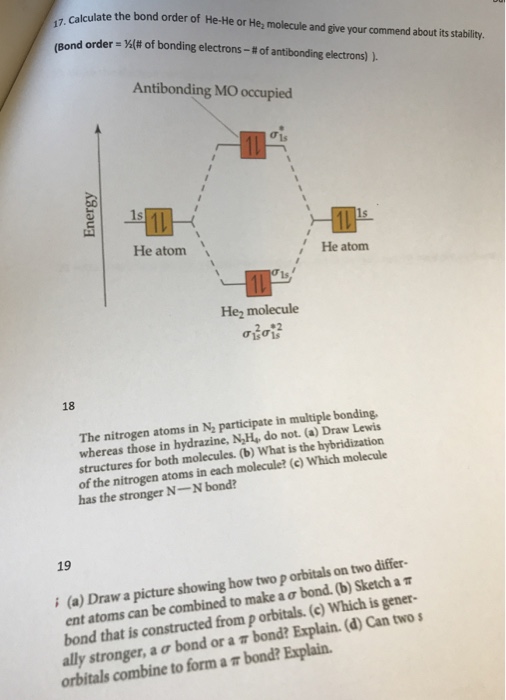
Solved Calculate The Bond Order Of HeHe Or He_2 Molecule...
To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. Bond order is 0.5, and. [2] this chemical is the largest diatomic. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. The he2+ ion is formed by removing two.
bond order of He_2^+ is
Here’s the best way to solve it. The he2+ ion is formed by removing two. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. Draw an mo diagram to predict.
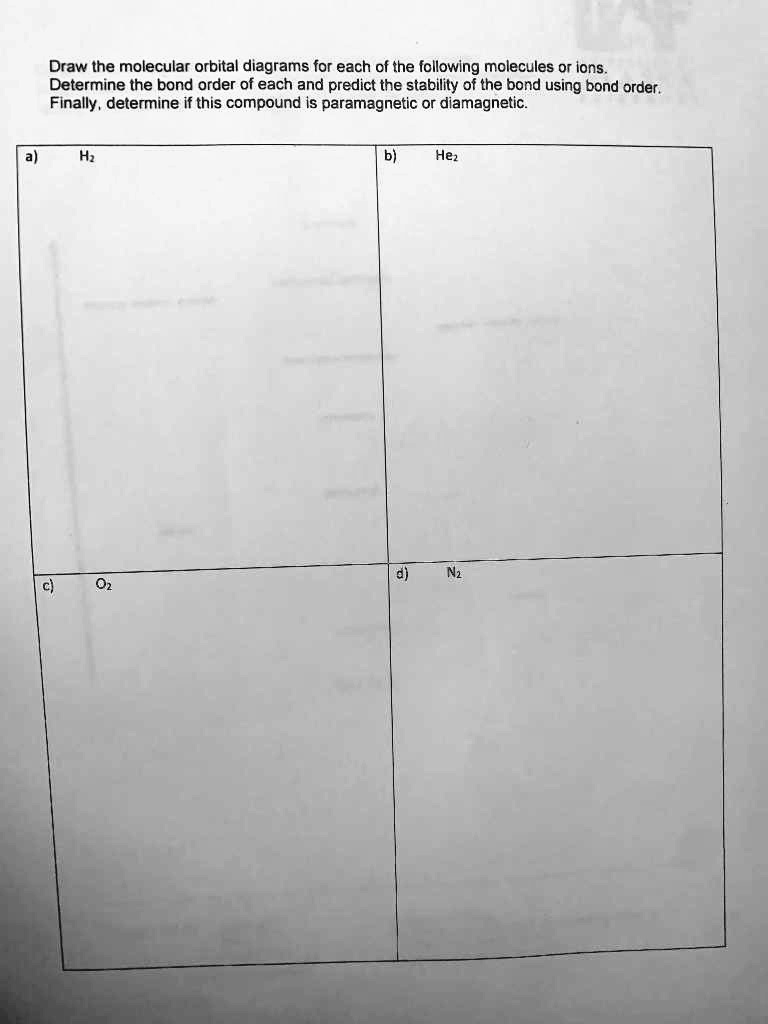
SOLVED Draw the molecular orbital diagrams for each of the following
The he2+ ion is formed by removing two. This means that the bond between the two. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. Bond order is 0.5, and. The helium dimer is a van der waals molecule with formula he 2 consisting of.
Solved Construct the molecular orbital diagram for H2 and
The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+.
This Means That The Bond Between The Two.
The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. Bond order is 0.5, and. [2] this chemical is the largest diatomic.
Draw An Mo Diagram To Predict The Bond Order And Stability Of An He2+ Molecule Formed From One He Atom And One He+ Ion.
Here’s the best way to solve it. The he2+ ion is formed by removing two. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by.