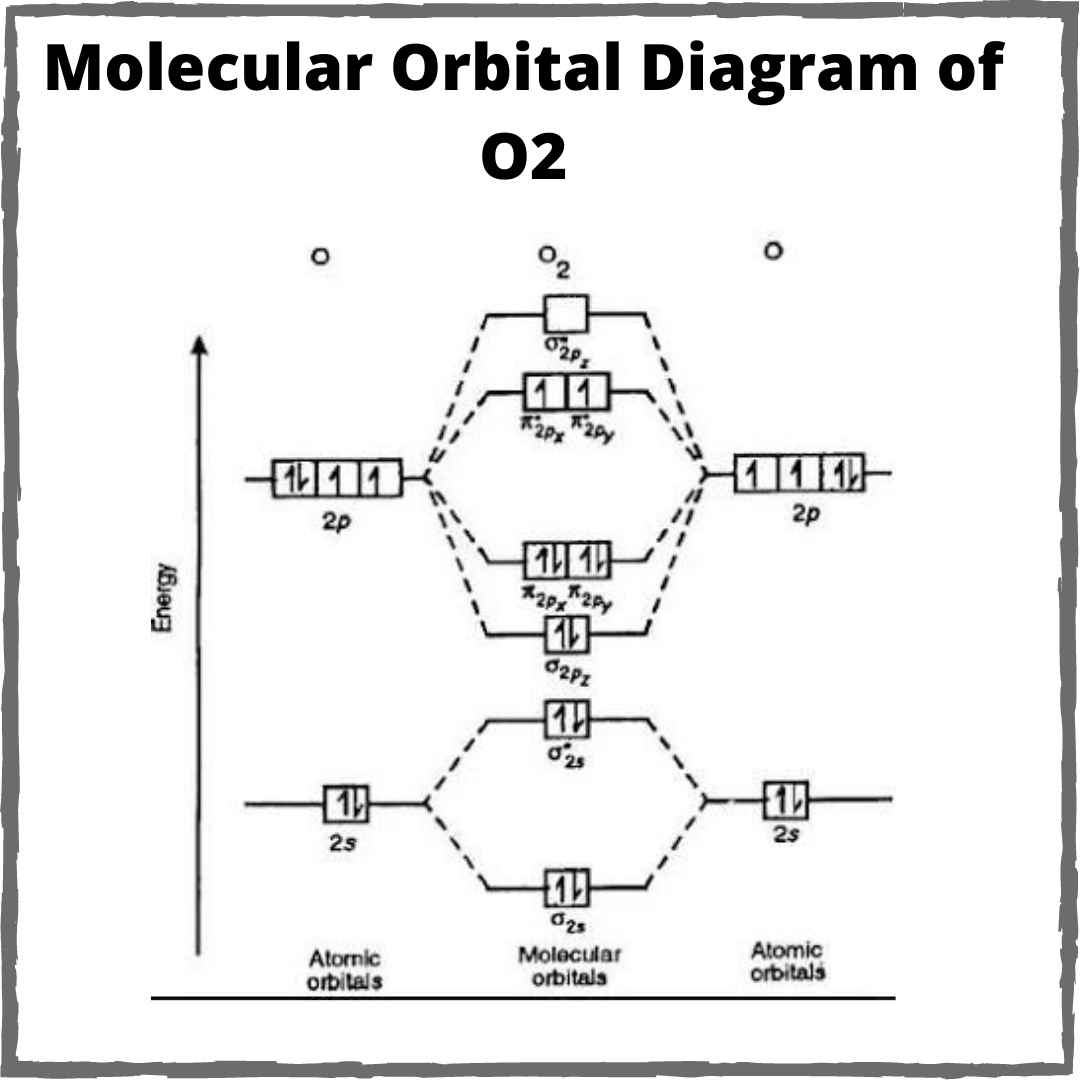
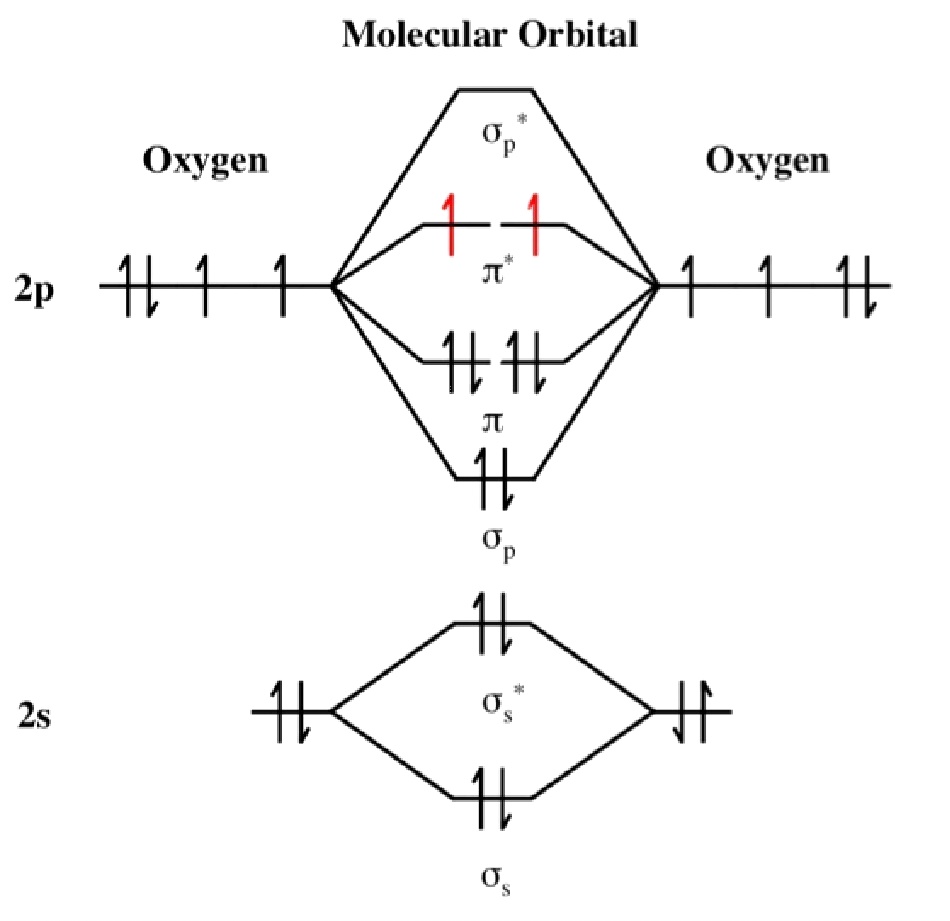
What Is The Bond Order Of O2
What Is The Bond Order Of O2 - Bond order is associated with the strength of bond and bond length. The bond order of o2+ is 2.5, indicating that it has a stable bond that is stronger than that of o2 (which has a bond order of 2). What is the bond order in o2 +? Remember that the bond order is the number of bonds between two atoms. A bond order of 1 indicates a stable molecule, as there is one net bonding electron. Over o2⁻, o2⁺ is more stable. O 2 has 16 electrons. O 2+ loses one electron, leaving 15 electrons. O2 has a bond order of 2 and two unpaired electrons in its π* orbitals. The higher the bond order, the smaller will be the bond length.
O 2+ loses one electron, leaving 15 electrons. Remember that the bond order is the number of bonds between two atoms. Bond order is associated with the strength of bond and bond length. A bond order of 1 indicates a stable molecule, as there is one net bonding electron. The higher the bond order, the smaller will be the bond length. The bond order of o2 is 1. Over o2⁻, o2⁺ is more stable. What is the bond order in o2 +? The bond order of o2+ is 2.5, indicating that it has a stable bond that is stronger than that of o2 (which has a bond order of 2). O 2 has 16 electrons.
What is the bond order in o2 +? O 2 has 16 electrons. A bond order of 1 indicates a stable molecule, as there is one net bonding electron. The higher the bond order, the smaller will be the bond length. O2 has a bond order of 2 and two unpaired electrons in its π* orbitals. Bond order is associated with the strength of bond and bond length. Remember that the bond order is the number of bonds between two atoms. The bond order of o2+ is 2.5, indicating that it has a stable bond that is stronger than that of o2 (which has a bond order of 2). The bond order of o2 is 1. O 2+ loses one electron, leaving 15 electrons.
Bond Order Of N2 astonishingceiyrs
Over o2⁻, o2⁺ is more stable. O 2 has 16 electrons. O 2+ loses one electron, leaving 15 electrons. A bond order of 1 indicates a stable molecule, as there is one net bonding electron. The bond order of o2+ is 2.5, indicating that it has a stable bond that is stronger than that of o2 (which has a bond.
The bond order of O_2^+ is the same as in
The higher the bond order, the smaller will be the bond length. Remember that the bond order is the number of bonds between two atoms. What is the bond order in o2 +? The bond order of o2+ is 2.5, indicating that it has a stable bond that is stronger than that of o2 (which has a bond order of.
Understanding the Bond Order in O2 through Molecular Orbital Diagrams
Bond order is associated with the strength of bond and bond length. The higher the bond order, the smaller will be the bond length. A bond order of 1 indicates a stable molecule, as there is one net bonding electron. Remember that the bond order is the number of bonds between two atoms. O 2+ loses one electron, leaving 15.
Calculate the bond order of N2,O2,O2^+and O2^ Chemistry Chemical
The higher the bond order, the smaller will be the bond length. Bond order is associated with the strength of bond and bond length. Remember that the bond order is the number of bonds between two atoms. The bond order of o2 is 1. O 2 has 16 electrons.
O2 Bond Order Diagram
Remember that the bond order is the number of bonds between two atoms. O2 has a bond order of 2 and two unpaired electrons in its π* orbitals. The bond order of o2 is 1. A bond order of 1 indicates a stable molecule, as there is one net bonding electron. The bond order of o2+ is 2.5, indicating that.
Explain why the bond order of N2 is greater than N2+, but the bond
What is the bond order in o2 +? The higher the bond order, the smaller will be the bond length. Over o2⁻, o2⁺ is more stable. The bond order of o2+ is 2.5, indicating that it has a stable bond that is stronger than that of o2 (which has a bond order of 2). O2 has a bond order of.
Calculate the bond order in O_2,O_2^,O_2^{2} and O_2^+ molecule.
The bond order of o2 is 1. O 2+ loses one electron, leaving 15 electrons. O2 has a bond order of 2 and two unpaired electrons in its π* orbitals. The bond order of o2+ is 2.5, indicating that it has a stable bond that is stronger than that of o2 (which has a bond order of 2). Bond order.
MO DIAGRAM of O2,O2,O2+ THEIR BOND ORDER AND CHAR
Bond order is associated with the strength of bond and bond length. O2 has a bond order of 2 and two unpaired electrons in its π* orbitals. The higher the bond order, the smaller will be the bond length. The bond order of o2 is 1. O 2 has 16 electrons.
O2 Bond Order Diagram
O 2+ loses one electron, leaving 15 electrons. What is the bond order in o2 +? Bond order is associated with the strength of bond and bond length. Remember that the bond order is the number of bonds between two atoms. Over o2⁻, o2⁺ is more stable.
What is meant by term bond order? Write bond orders for N2, O2?
Remember that the bond order is the number of bonds between two atoms. O 2 has 16 electrons. O2 has a bond order of 2 and two unpaired electrons in its π* orbitals. A bond order of 1 indicates a stable molecule, as there is one net bonding electron. O 2+ loses one electron, leaving 15 electrons.
A Bond Order Of 1 Indicates A Stable Molecule, As There Is One Net Bonding Electron.
The higher the bond order, the smaller will be the bond length. O 2 has 16 electrons. Bond order is associated with the strength of bond and bond length. The bond order of o2+ is 2.5, indicating that it has a stable bond that is stronger than that of o2 (which has a bond order of 2).
O 2+ Loses One Electron, Leaving 15 Electrons.
Over o2⁻, o2⁺ is more stable. O2 has a bond order of 2 and two unpaired electrons in its π* orbitals. Remember that the bond order is the number of bonds between two atoms. What is the bond order in o2 +?