What Is The Conjugate Acid Of Co3 2
What Is The Conjugate Acid Of Co3 2 - In this case, the conjugate base is the carbonate ion, co₃⁻². It is formed by accepting a proton (h+) to form carbonic acid (h2co3). A conjugate acid is a conjugate base with hydrogen ions attached to it. To find the conjugate acid, just add h +.
To find the conjugate acid, just add h +. A conjugate acid is a conjugate base with hydrogen ions attached to it. In this case, the conjugate base is the carbonate ion, co₃⁻². It is formed by accepting a proton (h+) to form carbonic acid (h2co3).
To find the conjugate acid, just add h +. A conjugate acid is a conjugate base with hydrogen ions attached to it. In this case, the conjugate base is the carbonate ion, co₃⁻². It is formed by accepting a proton (h+) to form carbonic acid (h2co3).
Conjugate AcidBase Pairs
A conjugate acid is a conjugate base with hydrogen ions attached to it. In this case, the conjugate base is the carbonate ion, co₃⁻². It is formed by accepting a proton (h+) to form carbonic acid (h2co3). To find the conjugate acid, just add h +.
Conjugate Acid Base Pair LarryecLloyd
In this case, the conjugate base is the carbonate ion, co₃⁻². It is formed by accepting a proton (h+) to form carbonic acid (h2co3). To find the conjugate acid, just add h +. A conjugate acid is a conjugate base with hydrogen ions attached to it.
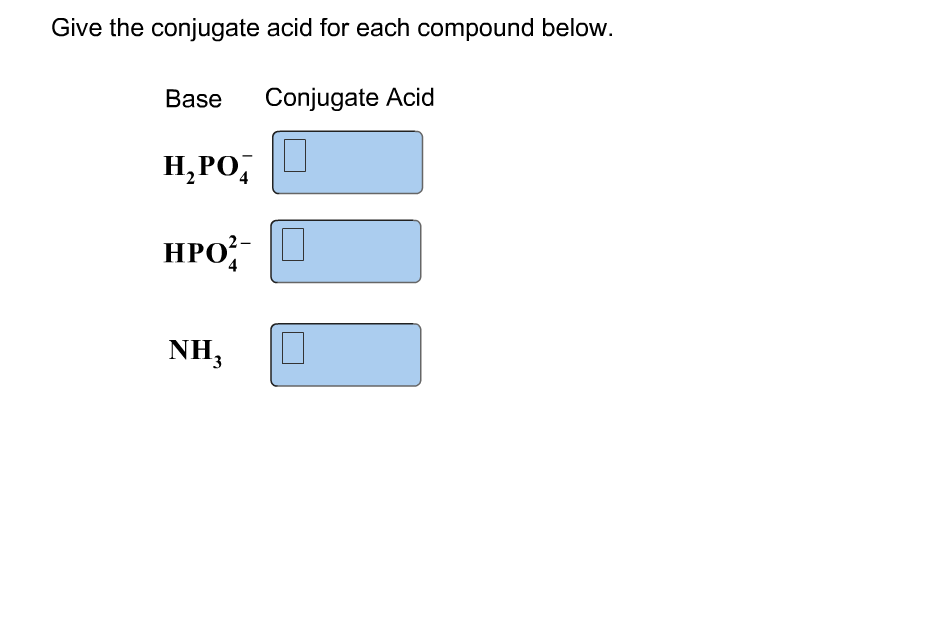
Solved Give the conjugate acid for each compound below Base
A conjugate acid is a conjugate base with hydrogen ions attached to it. To find the conjugate acid, just add h +. It is formed by accepting a proton (h+) to form carbonic acid (h2co3). In this case, the conjugate base is the carbonate ion, co₃⁻².
SOLVED Consider the following reaction NOz" HCO3 Lia CO3 2 + HNO2
In this case, the conjugate base is the carbonate ion, co₃⁻². To find the conjugate acid, just add h +. It is formed by accepting a proton (h+) to form carbonic acid (h2co3). A conjugate acid is a conjugate base with hydrogen ions attached to it.
[Solved] Part 2 Identifying Acid and Conjugate Base Ident
To find the conjugate acid, just add h +. A conjugate acid is a conjugate base with hydrogen ions attached to it. In this case, the conjugate base is the carbonate ion, co₃⁻². It is formed by accepting a proton (h+) to form carbonic acid (h2co3).
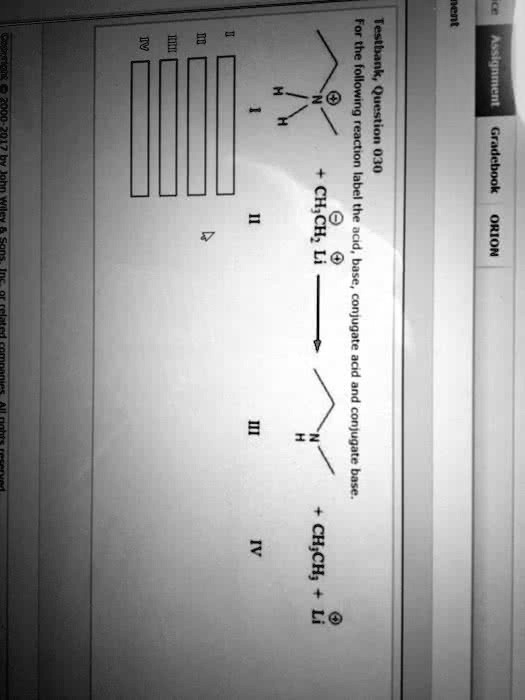
SOLVED ' For the following reaction label the acid, base, conjugate
To find the conjugate acid, just add h +. It is formed by accepting a proton (h+) to form carbonic acid (h2co3). A conjugate acid is a conjugate base with hydrogen ions attached to it. In this case, the conjugate base is the carbonate ion, co₃⁻².
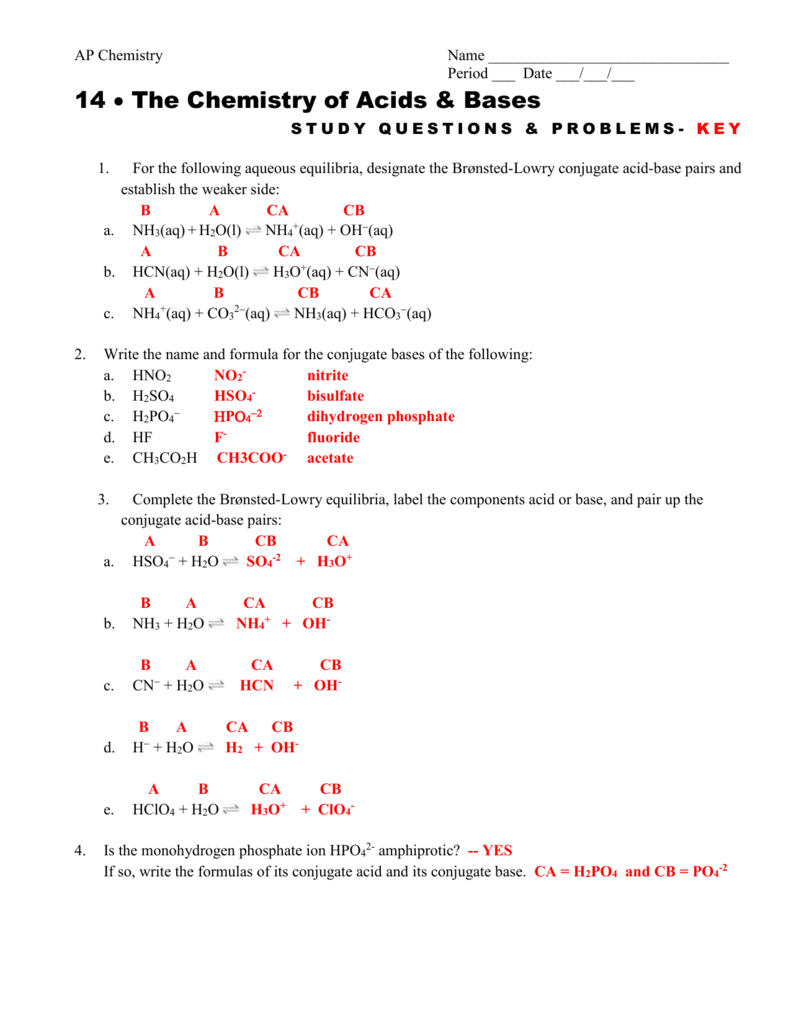
Conjugate Acid Base Pairs Worksheet Answers
A conjugate acid is a conjugate base with hydrogen ions attached to it. In this case, the conjugate base is the carbonate ion, co₃⁻². It is formed by accepting a proton (h+) to form carbonic acid (h2co3). To find the conjugate acid, just add h +.
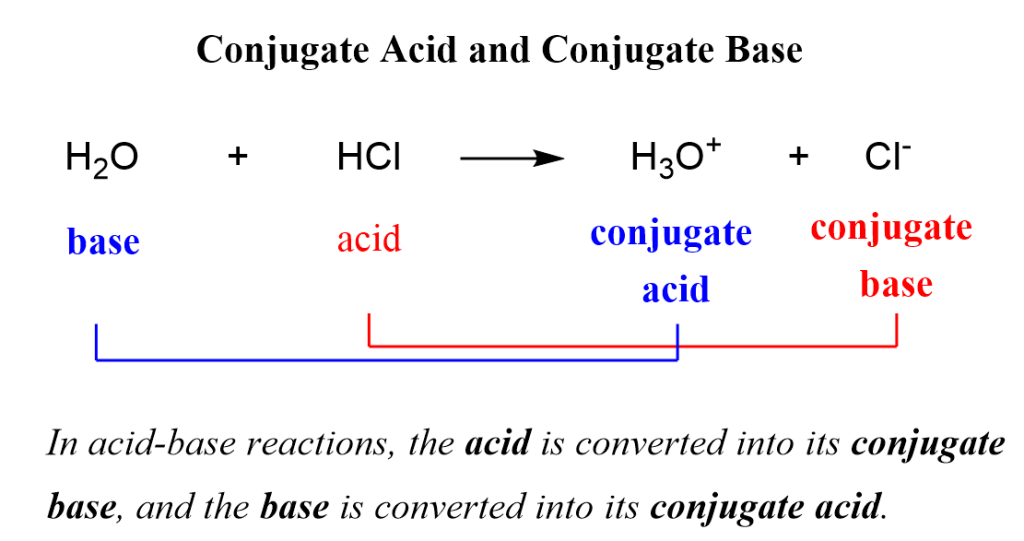
Conjugate Acid and Conjugate Base Chemistry Steps
In this case, the conjugate base is the carbonate ion, co₃⁻². To find the conjugate acid, just add h +. A conjugate acid is a conjugate base with hydrogen ions attached to it. It is formed by accepting a proton (h+) to form carbonic acid (h2co3).
Solved Identify acidbase conjugate pairs. (a) What is the
It is formed by accepting a proton (h+) to form carbonic acid (h2co3). A conjugate acid is a conjugate base with hydrogen ions attached to it. In this case, the conjugate base is the carbonate ion, co₃⁻². To find the conjugate acid, just add h +.
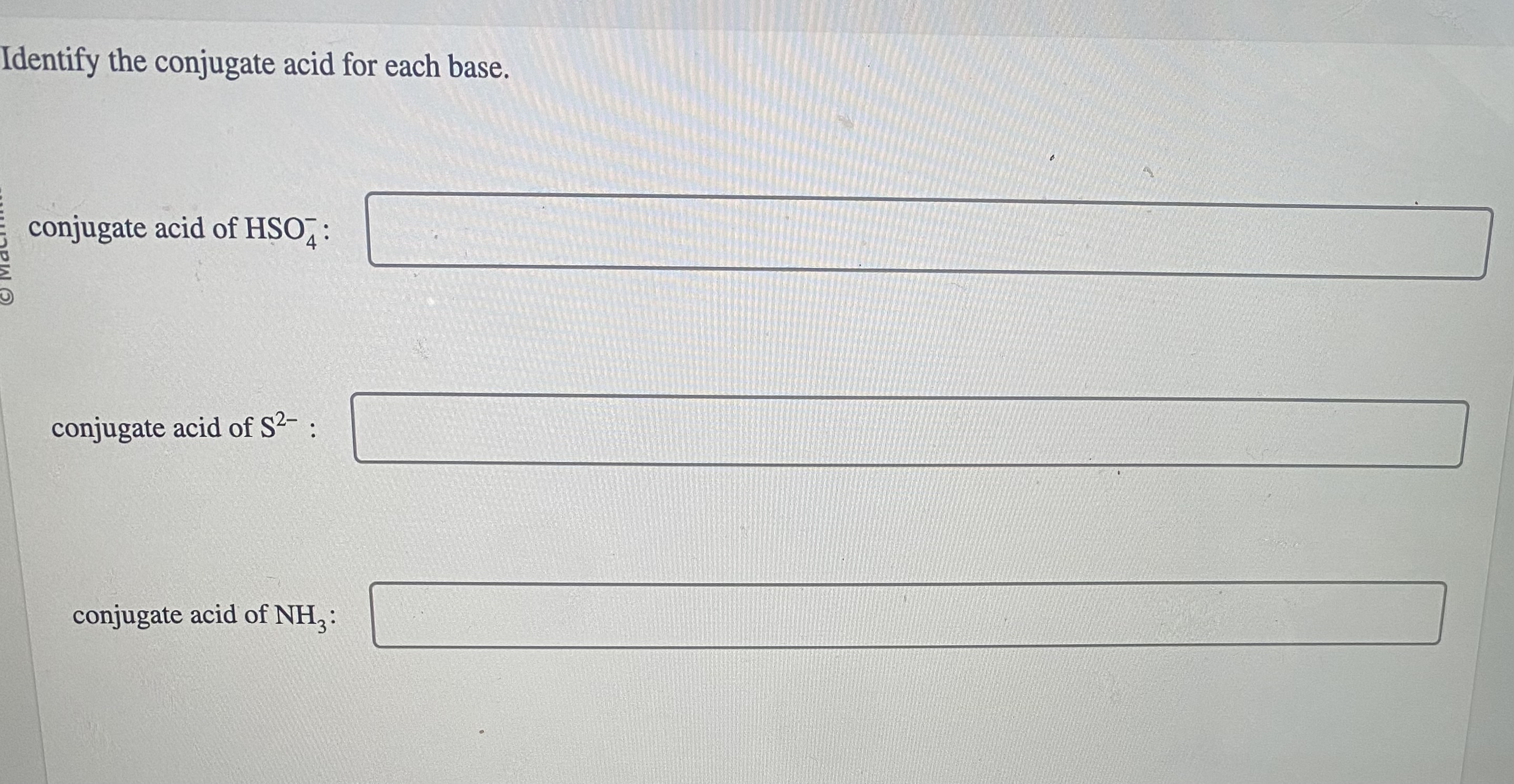
Solved Identify the conjugate acid for each base. conjugate
To find the conjugate acid, just add h +. A conjugate acid is a conjugate base with hydrogen ions attached to it. It is formed by accepting a proton (h+) to form carbonic acid (h2co3). In this case, the conjugate base is the carbonate ion, co₃⁻².
In This Case, The Conjugate Base Is The Carbonate Ion, Co₃⁻².
It is formed by accepting a proton (h+) to form carbonic acid (h2co3). To find the conjugate acid, just add h +. A conjugate acid is a conjugate base with hydrogen ions attached to it.



![[Solved] Part 2 Identifying Acid and Conjugate Base Ident](https://media.cheggcdn.com/study/d91/d91b4bb4-d9a4-4014-a470-e5ae500d1f5c/image)