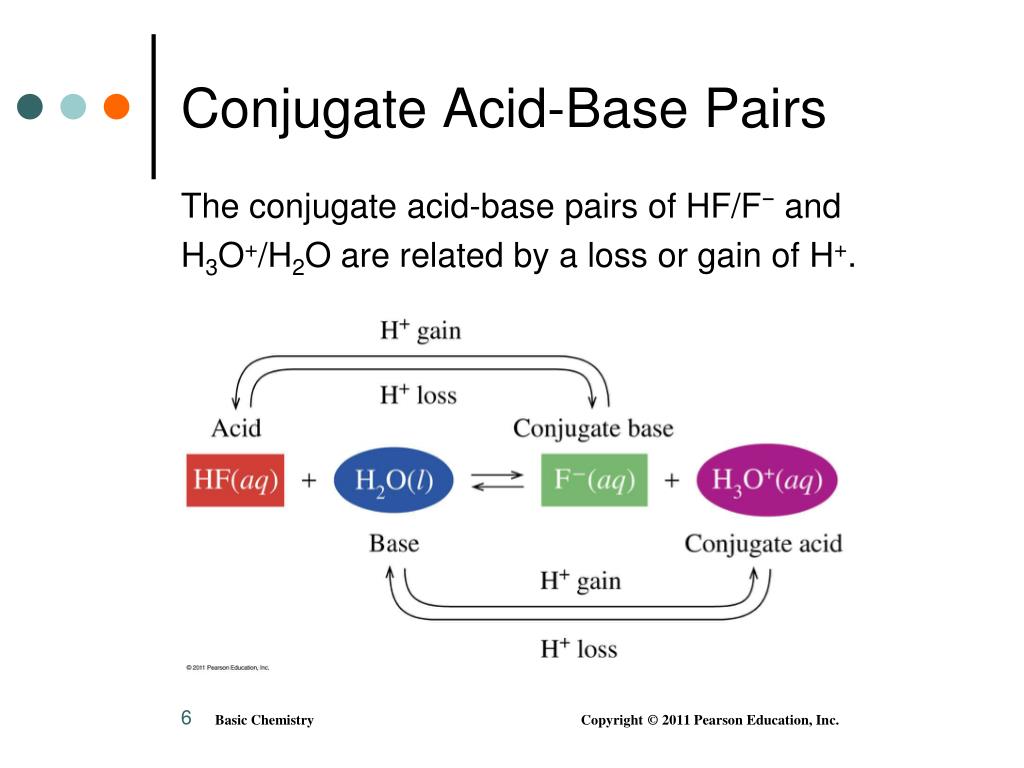
What Is The Conjugate Base Of H2Po4
What Is The Conjugate Base Of H2Po4 - This is because a conjugate base is formed when an acid donates a proton (h+). There are 3 steps to solve this one. Hence, conjugate base of h 2p o− 4 is h p o−2 4. The answer is option a) the conjugate base of an acid is the species formed after the. Therefore, conjugate base of h 2so4 is h so− 4. The loss of proton brings a negative charge on the compound and it's called the conjugate base of the acid.
Therefore, conjugate base of h 2so4 is h so− 4. Hence, conjugate base of h 2p o− 4 is h p o−2 4. This is because a conjugate base is formed when an acid donates a proton (h+). The loss of proton brings a negative charge on the compound and it's called the conjugate base of the acid. There are 3 steps to solve this one. The answer is option a) the conjugate base of an acid is the species formed after the.
The answer is option a) the conjugate base of an acid is the species formed after the. Therefore, conjugate base of h 2so4 is h so− 4. The loss of proton brings a negative charge on the compound and it's called the conjugate base of the acid. There are 3 steps to solve this one. Hence, conjugate base of h 2p o− 4 is h p o−2 4. This is because a conjugate base is formed when an acid donates a proton (h+).
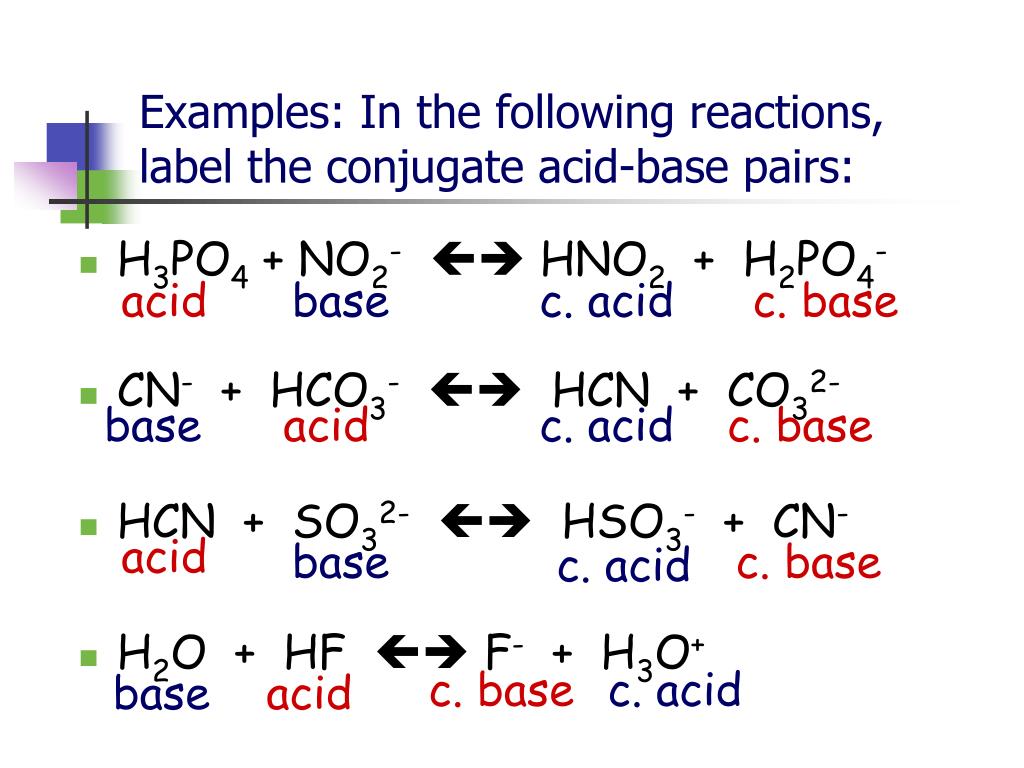
Conjugate Acid Vs Conjugate Base
Therefore, conjugate base of h 2so4 is h so− 4. There are 3 steps to solve this one. The loss of proton brings a negative charge on the compound and it's called the conjugate base of the acid. Hence, conjugate base of h 2p o− 4 is h p o−2 4. This is because a conjugate base is formed when.
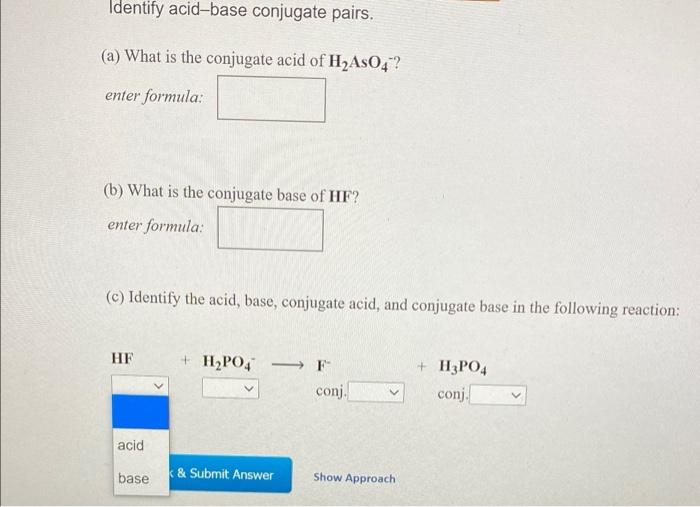
Solved Identify acidbase conjugate pairs. (a) What is the
This is because a conjugate base is formed when an acid donates a proton (h+). Therefore, conjugate base of h 2so4 is h so− 4. The loss of proton brings a negative charge on the compound and it's called the conjugate base of the acid. Hence, conjugate base of h 2p o− 4 is h p o−2 4. The answer.
Conjugate Base Of H3po4 Asking List
Therefore, conjugate base of h 2so4 is h so− 4. The answer is option a) the conjugate base of an acid is the species formed after the. This is because a conjugate base is formed when an acid donates a proton (h+). Hence, conjugate base of h 2p o− 4 is h p o−2 4. The loss of proton brings.
Conjugate Base Of H3po4 Asking List
This is because a conjugate base is formed when an acid donates a proton (h+). Therefore, conjugate base of h 2so4 is h so− 4. Hence, conjugate base of h 2p o− 4 is h p o−2 4. There are 3 steps to solve this one. The loss of proton brings a negative charge on the compound and it's called.
Conjugate Base Of H3po4 Asking List
Hence, conjugate base of h 2p o− 4 is h p o−2 4. There are 3 steps to solve this one. The answer is option a) the conjugate base of an acid is the species formed after the. Therefore, conjugate base of h 2so4 is h so− 4. This is because a conjugate base is formed when an acid donates.
Solved What are the conjugate base and conjugate acid of
Therefore, conjugate base of h 2so4 is h so− 4. The loss of proton brings a negative charge on the compound and it's called the conjugate base of the acid. This is because a conjugate base is formed when an acid donates a proton (h+). Hence, conjugate base of h 2p o− 4 is h p o−2 4. The answer.
Conjugate Base Of H3po4 Asking List
This is because a conjugate base is formed when an acid donates a proton (h+). Hence, conjugate base of h 2p o− 4 is h p o−2 4. There are 3 steps to solve this one. Therefore, conjugate base of h 2so4 is h so− 4. The loss of proton brings a negative charge on the compound and it's called.
Conjugate Base Of H3po4 Asking List
The loss of proton brings a negative charge on the compound and it's called the conjugate base of the acid. This is because a conjugate base is formed when an acid donates a proton (h+). The answer is option a) the conjugate base of an acid is the species formed after the. Hence, conjugate base of h 2p o− 4.
Conjugate Base Of H2po4 Asking List
There are 3 steps to solve this one. Therefore, conjugate base of h 2so4 is h so− 4. Hence, conjugate base of h 2p o− 4 is h p o−2 4. This is because a conjugate base is formed when an acid donates a proton (h+). The loss of proton brings a negative charge on the compound and it's called.
H2PO4 Conjugate Base
The answer is option a) the conjugate base of an acid is the species formed after the. Hence, conjugate base of h 2p o− 4 is h p o−2 4. The loss of proton brings a negative charge on the compound and it's called the conjugate base of the acid. This is because a conjugate base is formed when an.
The Loss Of Proton Brings A Negative Charge On The Compound And It's Called The Conjugate Base Of The Acid.
Therefore, conjugate base of h 2so4 is h so− 4. There are 3 steps to solve this one. Hence, conjugate base of h 2p o− 4 is h p o−2 4. The answer is option a) the conjugate base of an acid is the species formed after the.