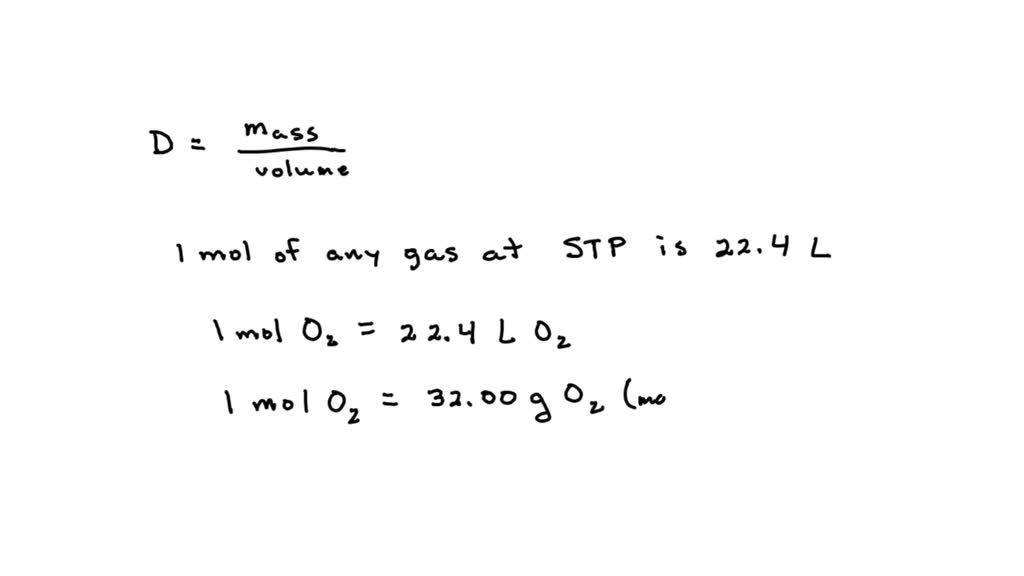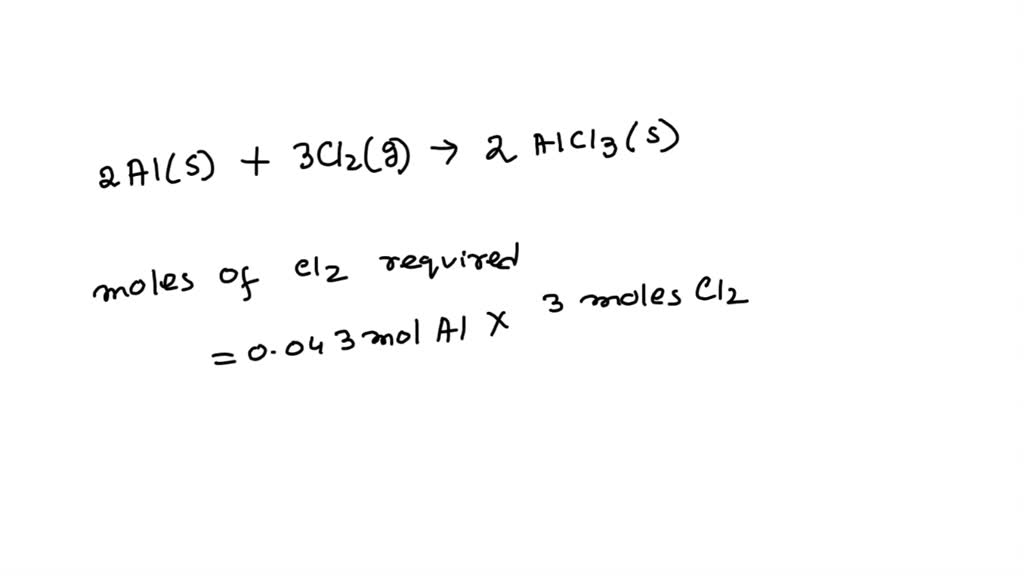What Is The Density Of Chlorine Gas At Stp
What Is The Density Of Chlorine Gas At Stp - The molar mass of chlorine gas (cl2) is. By plugging in the values for stp and. To calculate the density of chlorine at stp, we use the modified ideal gas law density equation, ρ = pm / rt. The molar mass of cl2 is 70.9 g. To calculate the density of chlorine gas, , at standard temperature and pressure (stp), we can follow these steps: The molar mass of chlorine gas (cl2) is. What is the density of chlorine gas at stp? 1 mole of a gas occupies 22.4 l of volume. The density of chlorine gas is 3.17 g/l. The molar volume of any gas at standard temperature and pressure (stp) is 22.4 l/mol.
What is the density of chlorine gas at stp? By plugging in the values for stp and. To calculate the density of chlorine at stp, we use the modified ideal gas law density equation, ρ = pm / rt. The molar mass of chlorine gas (cl2) is. This is a type of problem so we use the equation since the. The density of chlorine gas is 3.17 g/l. At standard temperature and pressure (stp), one mole of any gas occupies a volume of 22.4 liters. The molar volume of any gas at standard temperature and pressure (stp) is 22.4 l/mol. What is the density of a 1.50 g sample of chlorine gas, confined in a flask, that exerts a pressure of 735.0 mmhg at 25.0 c?. To calculate the density of chlorine gas, , at standard temperature and pressure (stp), we can follow these steps:
1 mole of a gas occupies 22.4 l of volume. At standard temperature and pressure (stp), one mole of any gas occupies a volume of 22.4 liters. To calculate the density of chlorine at stp, we use the modified ideal gas law density equation, ρ = pm / rt. The molar mass of chlorine gas (cl2) is. This is a type of problem so we use the equation since the. What is the density of a 1.50 g sample of chlorine gas, confined in a flask, that exerts a pressure of 735.0 mmhg at 25.0 c?. The molar mass of chlorine gas (cl2) is. By plugging in the values for stp and. To calculate the density of chlorine gas, , at standard temperature and pressure (stp), we can follow these steps: The density of chlorine gas is 3.17 g/l.
27. The density of a gas STP is 1.5g/L STP. Its molecular weight is 1
This is a type of problem so we use the equation since the. What is the density of chlorine gas at stp? The density of chlorine gas is 3.17 g/l. 1 mole of a gas occupies 22.4 l of volume. To calculate the density of chlorine gas, , at standard temperature and pressure (stp), we can follow these steps:
Calculate the density of oxygen gas at STP using the molar volume of
This is a type of problem so we use the equation since the. What is the density of chlorine gas at stp? The density of chlorine gas is 3.17 g/l. The molar mass of chlorine gas (cl2) is. The molar mass of cl2 is 70.9 g.
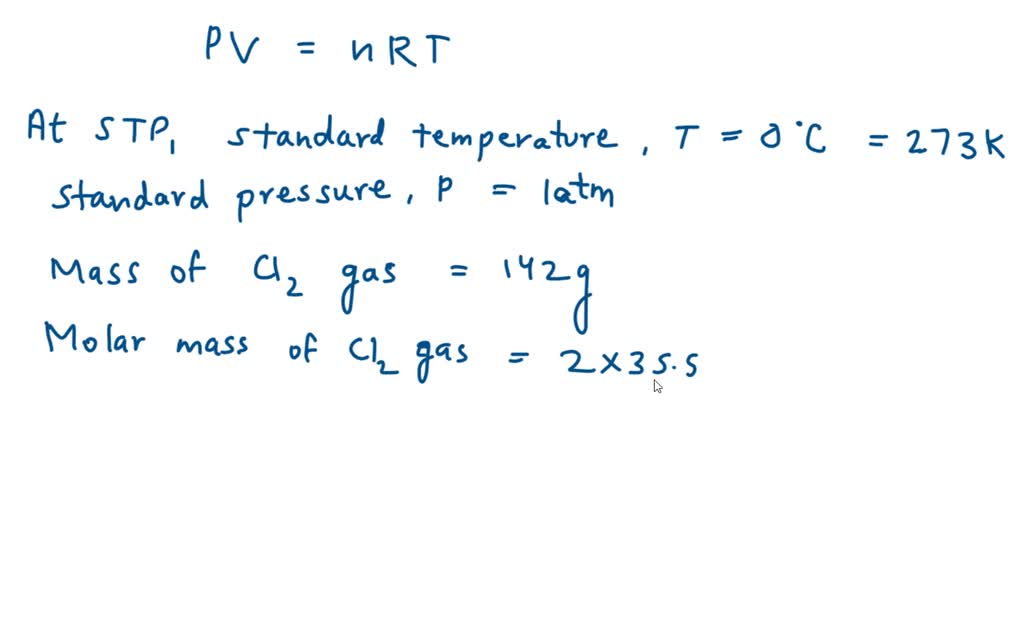
SOLVED Calculate the volume in litres of 142 g chlorine gas STP?
1 mole of a gas occupies 22.4 l of volume. The molar mass of cl2 is 70.9 g. The molar mass of chlorine gas (cl2) is. By plugging in the values for stp and. To calculate the density of chlorine gas, , at standard temperature and pressure (stp), we can follow these steps:
Chlorine Gas At Stp at Lorna Kerr blog
At standard temperature and pressure (stp), one mole of any gas occupies a volume of 22.4 liters. To calculate the density of chlorine at stp, we use the modified ideal gas law density equation, ρ = pm / rt. The molar volume of any gas at standard temperature and pressure (stp) is 22.4 l/mol. The molar mass of chlorine gas.
Vapour density=Density of gas at STP/Density of hydrogen gas at STP
What is the density of chlorine gas at stp? The molar mass of chlorine gas (cl2) is. The density of chlorine gas is 3.17 g/l. The molar volume of any gas at standard temperature and pressure (stp) is 22.4 l/mol. The molar mass of cl2 is 70.9 g.
Chlorine Gas At Stp at Lorna Kerr blog
The molar mass of chlorine gas (cl2) is. The molar mass of cl2 is 70.9 g. What is the density of chlorine gas at stp? By plugging in the values for stp and. The density of chlorine gas is 3.17 g/l.
Solved Use the molar volume of a gas at STP to calculate the
By plugging in the values for stp and. 1 mole of a gas occupies 22.4 l of volume. The molar volume of any gas at standard temperature and pressure (stp) is 22.4 l/mol. The density of chlorine gas is 3.17 g/l. At standard temperature and pressure (stp), one mole of any gas occupies a volume of 22.4 liters.
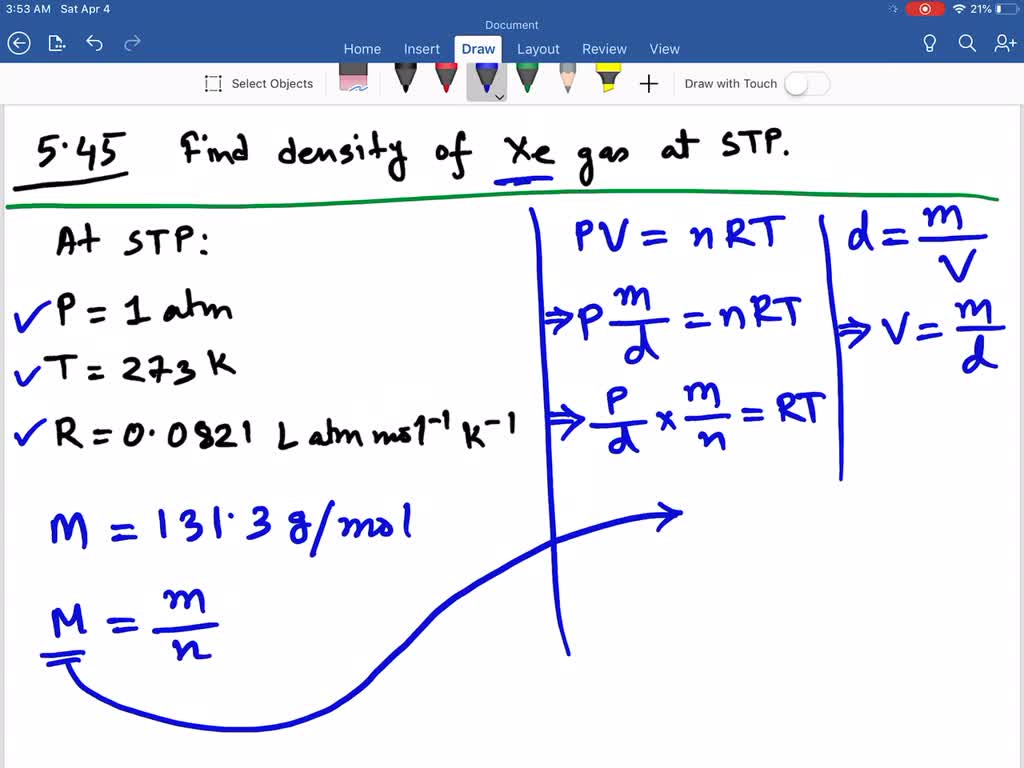
SOLVEDWhat is the density of Xe gas at STP?
The molar mass of chlorine gas (cl2) is. To calculate the density of chlorine at stp, we use the modified ideal gas law density equation, ρ = pm / rt. What is the density of chlorine gas at stp? 1 mole of a gas occupies 22.4 l of volume. The density of chlorine gas is 3.17 g/l.
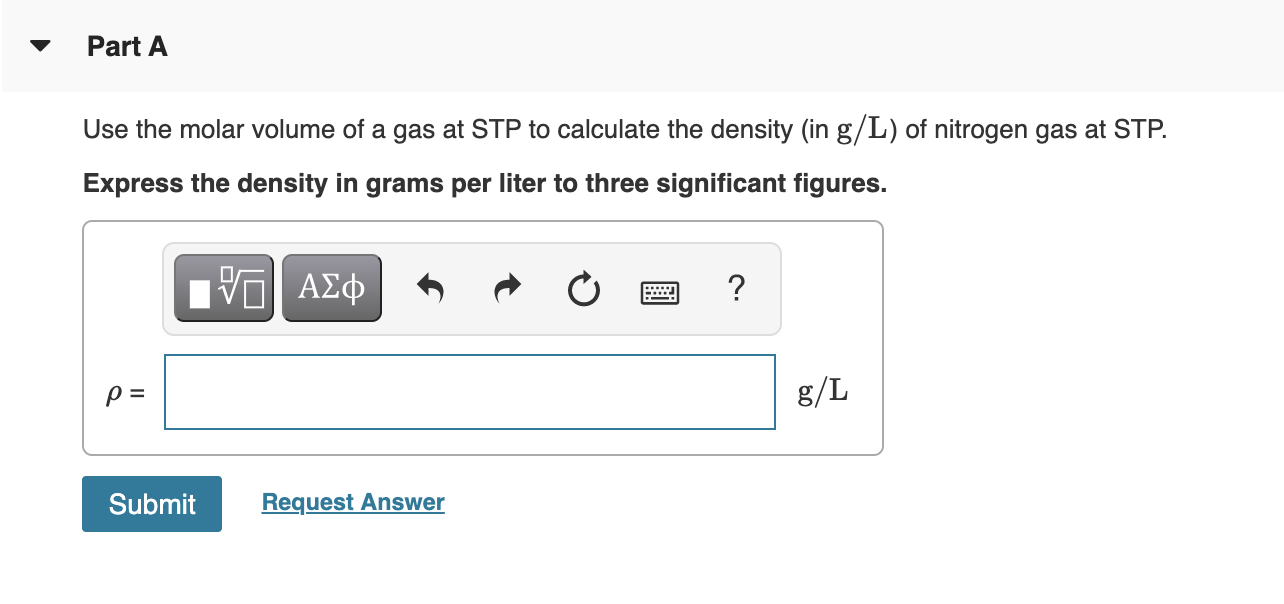
Solved Part A Use the molar volume of a gas at STP to
The molar volume of any gas at standard temperature and pressure (stp) is 22.4 l/mol. To calculate the density of chlorine gas, , at standard temperature and pressure (stp), we can follow these steps: By plugging in the values for stp and. The molar mass of cl2 is 70.9 g. What is the density of chlorine gas at stp?
SOLVED The density of chlorine gas at STP, in grams per liter, is
What is the density of a 1.50 g sample of chlorine gas, confined in a flask, that exerts a pressure of 735.0 mmhg at 25.0 c?. By plugging in the values for stp and. To calculate the density of chlorine gas, , at standard temperature and pressure (stp), we can follow these steps: The molar mass of chlorine gas (cl2).
This Is A Type Of Problem So We Use The Equation Since The.
To calculate the density of chlorine gas, , at standard temperature and pressure (stp), we can follow these steps: The molar mass of chlorine gas (cl2) is. The density of chlorine gas is 3.17 g/l. By plugging in the values for stp and.
1 Mole Of A Gas Occupies 22.4 L Of Volume.
The molar mass of chlorine gas (cl2) is. The molar volume of any gas at standard temperature and pressure (stp) is 22.4 l/mol. At standard temperature and pressure (stp), one mole of any gas occupies a volume of 22.4 liters. To calculate the density of chlorine at stp, we use the modified ideal gas law density equation, ρ = pm / rt.
What Is The Density Of A 1.50 G Sample Of Chlorine Gas, Confined In A Flask, That Exerts A Pressure Of 735.0 Mmhg At 25.0 C?.
What is the density of chlorine gas at stp? The molar mass of cl2 is 70.9 g.