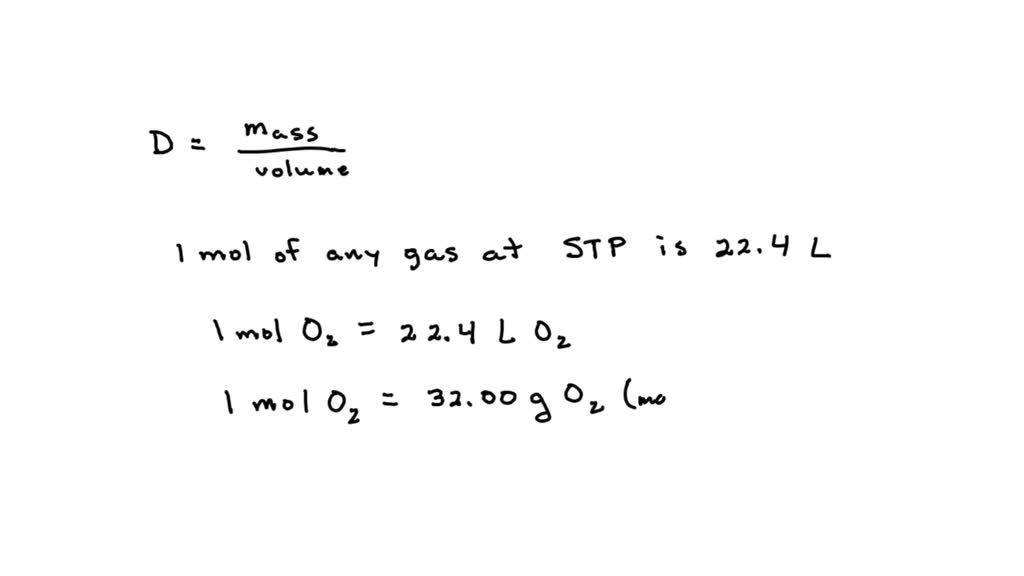What Is The Density Of Krypton Gas At Stp
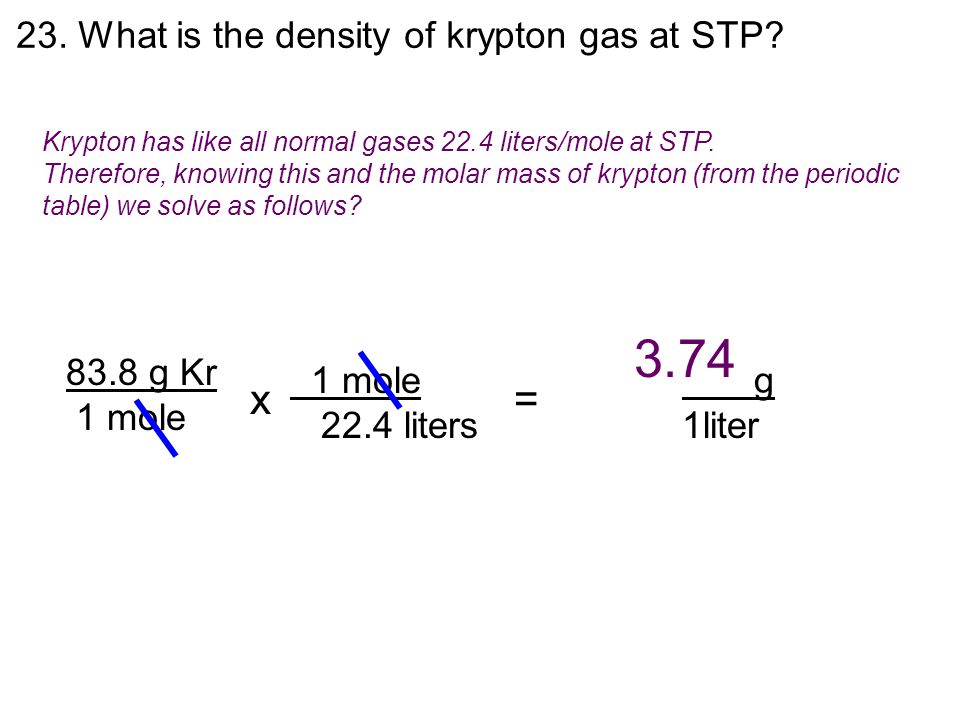
What Is The Density Of Krypton Gas At Stp - Pv = nrt, where p is pressure, v is volume, n is the. Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. The density of a gas at stp can be calculated using the ideal gas law: How do you find the density of krypton? The equation used to calculate the density is:
The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. How do you find the density of krypton? The equation used to calculate the density is: Pv = nrt, where p is pressure, v is volume, n is the. Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of a gas at stp can be calculated using the ideal gas law: The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c.
The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. How do you find the density of krypton? Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of a gas at stp can be calculated using the ideal gas law: Pv = nrt, where p is pressure, v is volume, n is the. The equation used to calculate the density is: The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1.
What Is the Density of Krypton Gas at Stp
The equation used to calculate the density is: Pv = nrt, where p is pressure, v is volume, n is the. The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. The density of a gas at stp can be calculated using the ideal gas law: Density = mass /.
27. The density of a gas STP is 1.5g/L STP. Its molecular weight is 1
Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The equation used to calculate the density is: The density of a gas at stp can be calculated using the ideal gas law: How do you find the density of krypton? The density of an ideal gas at standard temperature and pressure (stp), which is.
What Is the Density of Krypton Gas at Stp
How do you find the density of krypton? The equation used to calculate the density is: Pv = nrt, where p is pressure, v is volume, n is the. The density of a gas at stp can be calculated using the ideal gas law: The density of an ideal gas at standard temperature and pressure (stp), which is defined as.
What Is the Density of Krypton Gas at Stp
How do you find the density of krypton? The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The equation used to calculate the density is: The density of a gas at stp can be.
What Is the Density of Krypton Gas at Stp
Pv = nrt, where p is pressure, v is volume, n is the. The equation used to calculate the density is: The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. How do you find the density of krypton? The density of a gas at stp can be calculated.
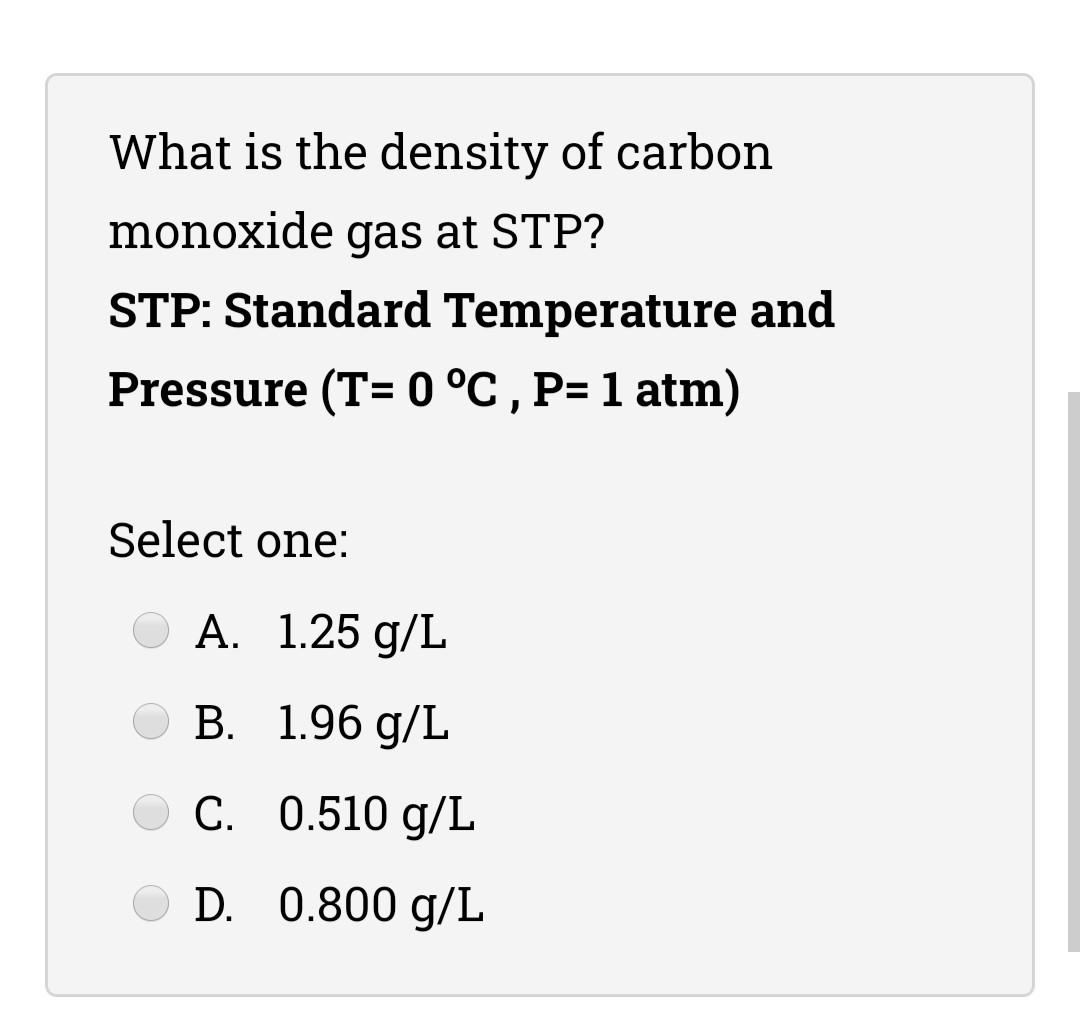
(Solved) What Is The Density Of Carbon Monoxide Gas At STP? STP
Density = mass / volume mass of krypton = 83.798 g the volume occupied any. Pv = nrt, where p is pressure, v is volume, n is the. The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. The equation used to calculate the density is: How do you find.
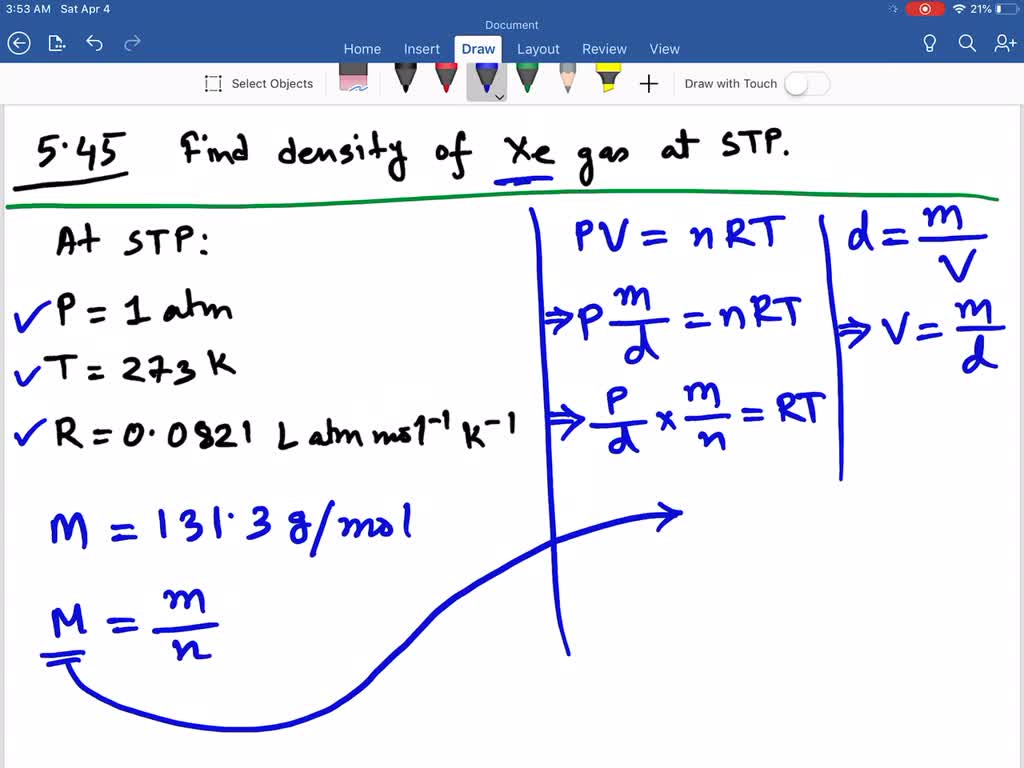
SOLVEDWhat is the density of Xe gas at STP?
The density of a gas at stp can be calculated using the ideal gas law: Pv = nrt, where p is pressure, v is volume, n is the. How do you find the density of krypton? The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. The equation used.
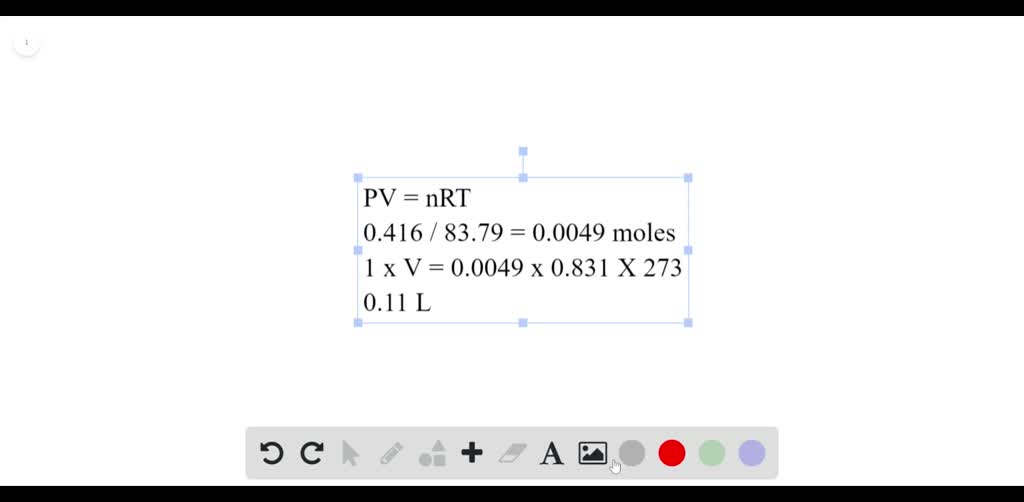
What volume will 0.416 g of krypton gas occupy at STP? Numerade
How do you find the density of krypton? Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of a gas at stp can be calculated using the ideal gas law: The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. Pv =.
Solved A sample of Krypton gas at STP occupies 40.1 liters.
Density = mass / volume mass of krypton = 83.798 g the volume occupied any. How do you find the density of krypton? The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. Pv = nrt, where p is pressure, v is volume, n is the. The equation used to.
Calculate the density of oxygen gas at STP using the molar volume of
Pv = nrt, where p is pressure, v is volume, n is the. How do you find the density of krypton? The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature.
The Density Of An Ideal Gas At Standard Temperature And Pressure (Stp), Which Is Defined As A Temperature Of 0°C.
The equation used to calculate the density is: The density of a gas at stp can be calculated using the ideal gas law: The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. Pv = nrt, where p is pressure, v is volume, n is the.
How Do You Find The Density Of Krypton?
Density = mass / volume mass of krypton = 83.798 g the volume occupied any.