What Is The Effective Nuclear Charge Of Nitrogen
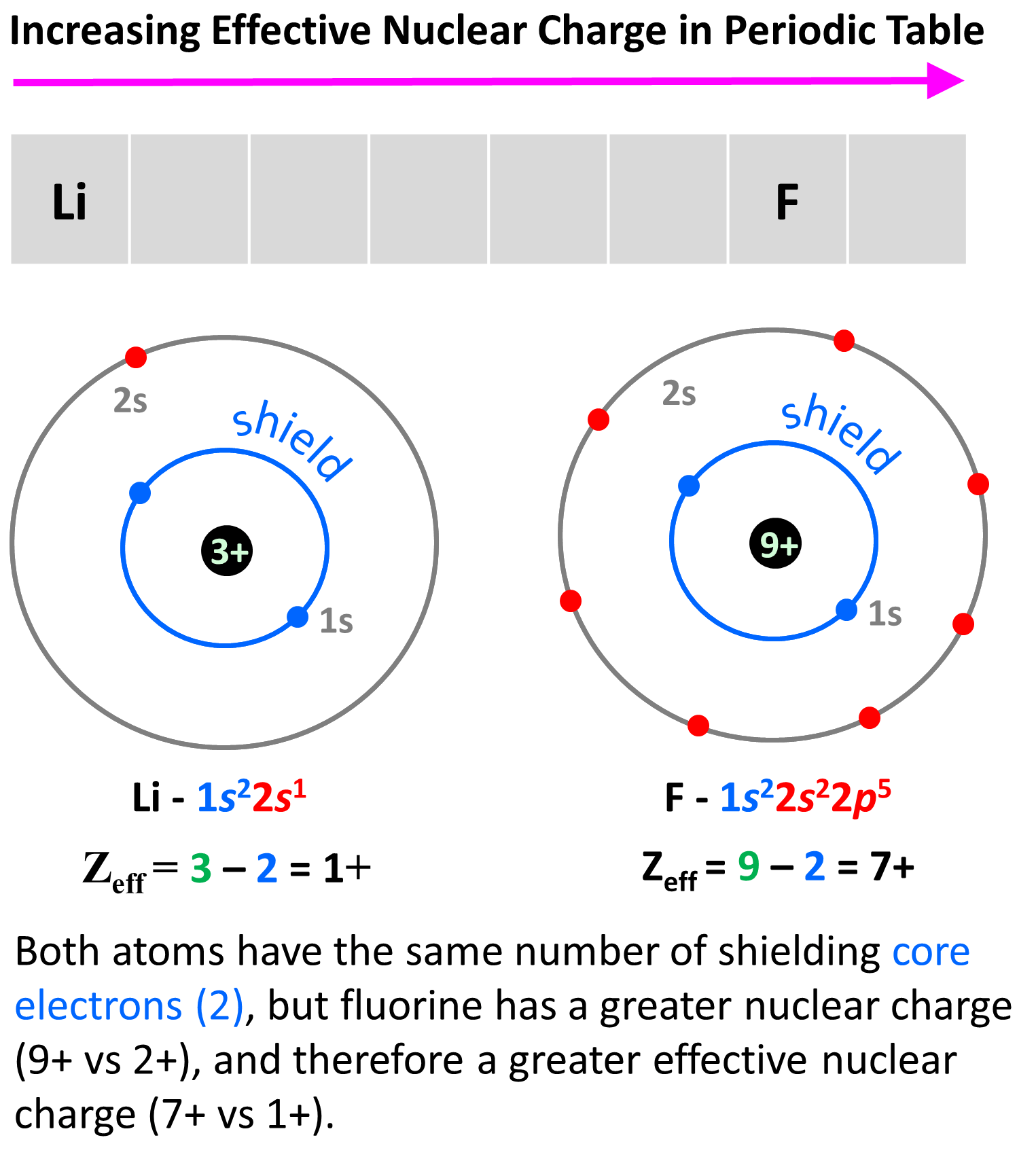
What Is The Effective Nuclear Charge Of Nitrogen - The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.). Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion.
The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.). Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion.
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.).
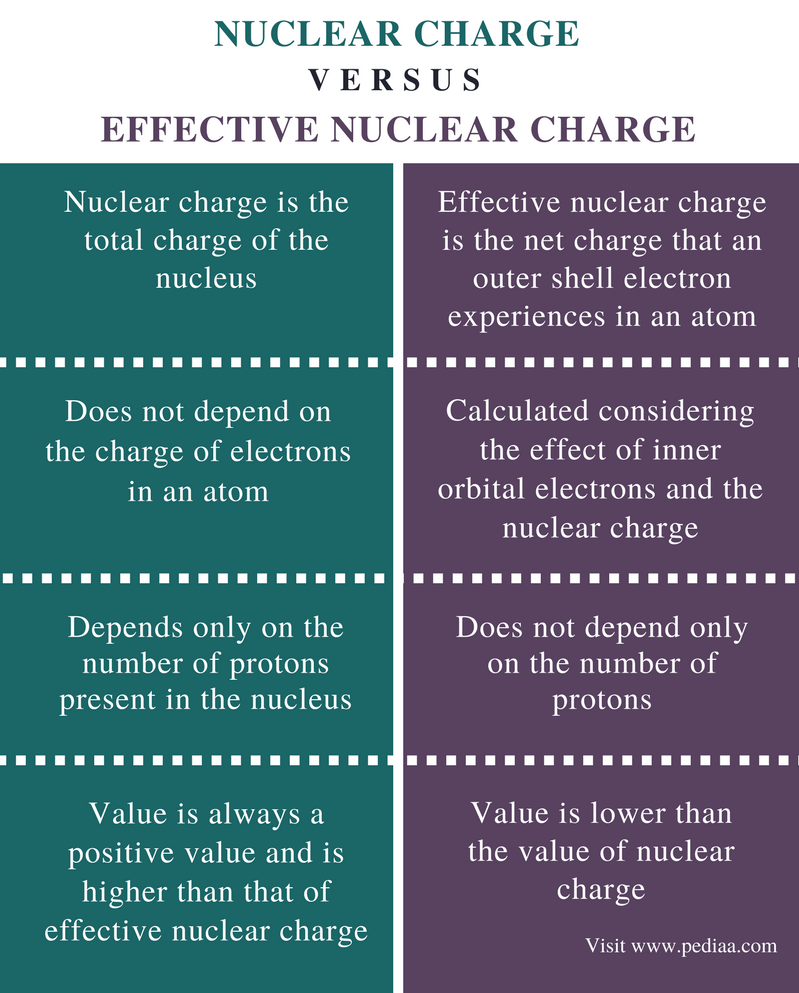
Difference Between Nuclear Charge and Effective Nuclear Charge
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.).
Effective Nuclear Charge Chemistry Steps
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.).
Effective Nuclear Charge Chemistry Steps
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.).
Understanding Effective Nuclear Charge Sunaan
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.).
Effective Nuclear Charge Chemistry Steps
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.).
Effective Nuclear Charge Chemistry Steps
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.).
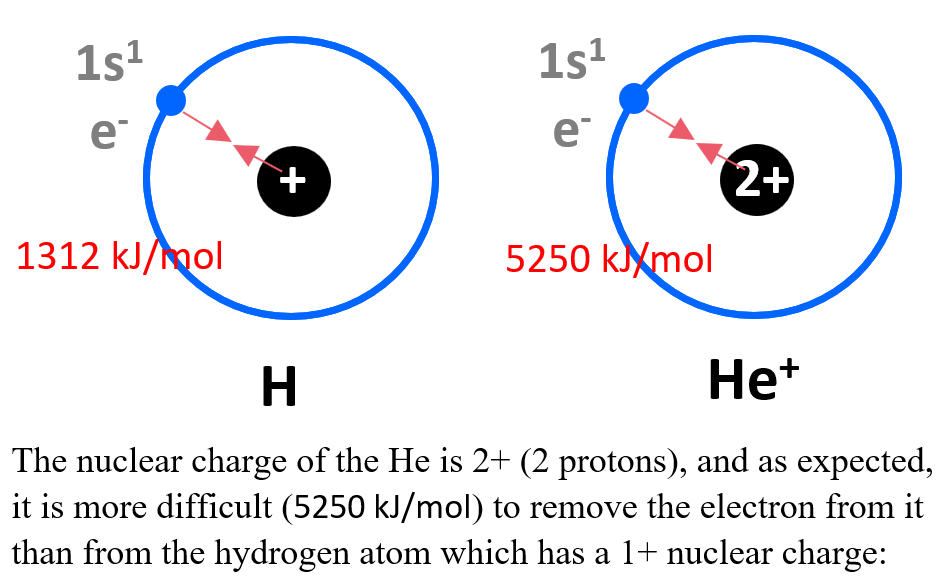
Effective Nuclear Charge
The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.). Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion.
Effective Nuclear Charge Definition, Formula, and Chart
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.).
What is Effective Nuclear Charge?
The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.). Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion.
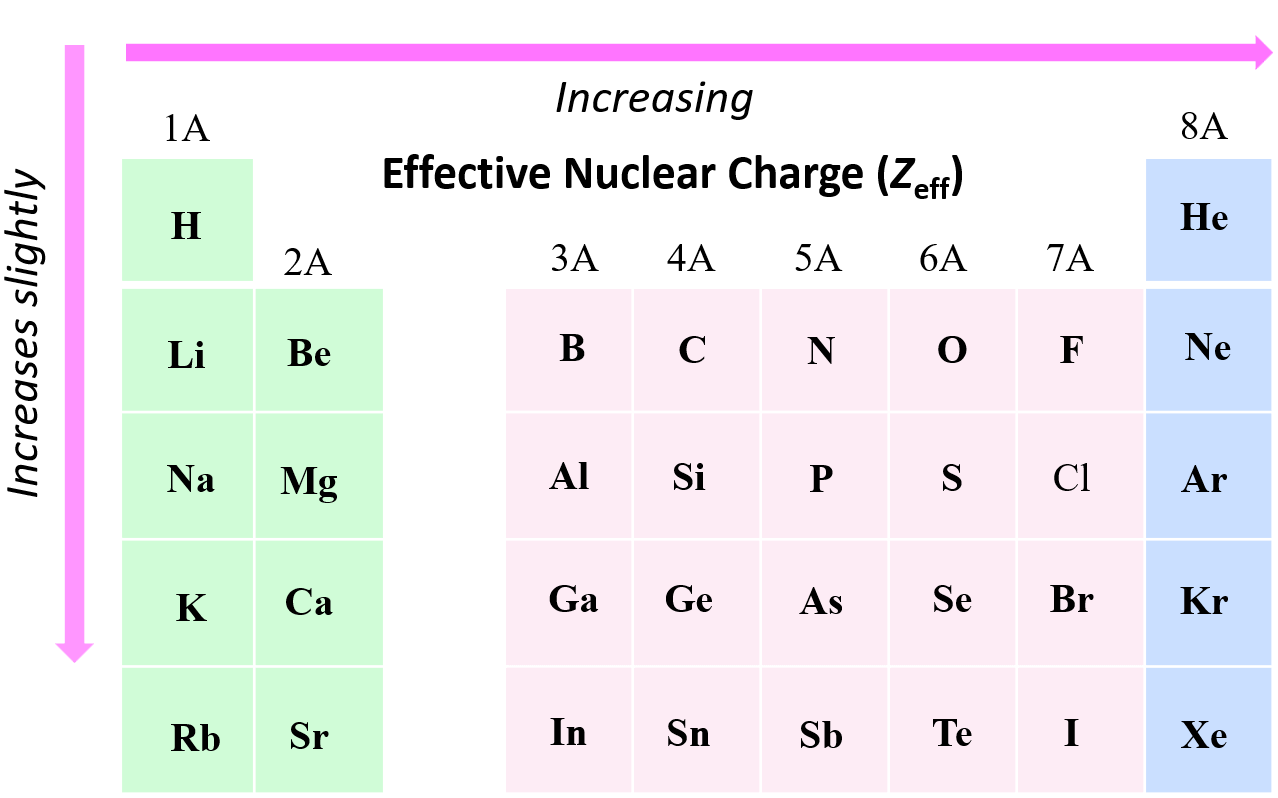
Effective Nuclear Charge Trend
The effective nuclear charge is determined by subtracting from the number of protons in the nucleus (z), the number of inner core (i.c.). Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion.
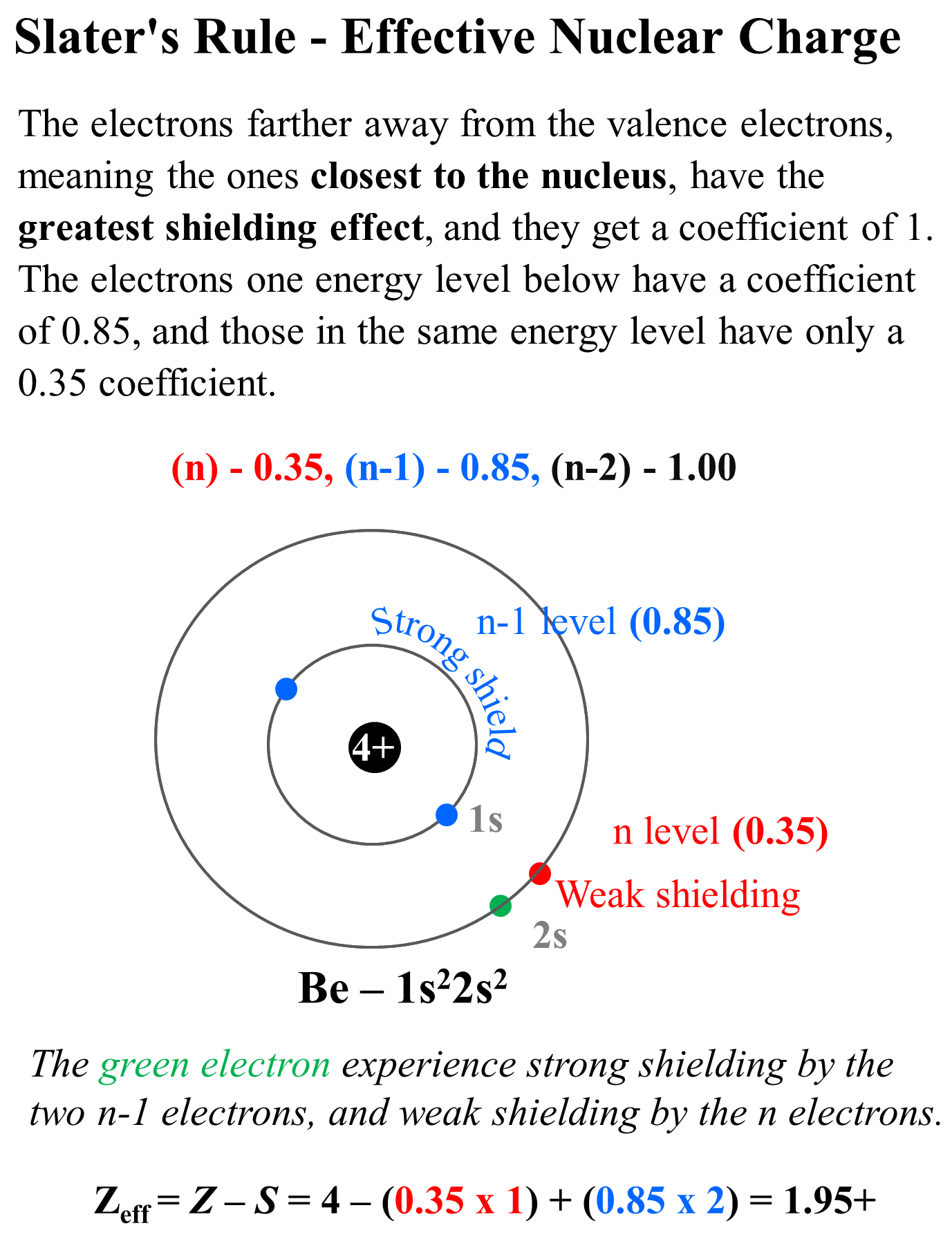
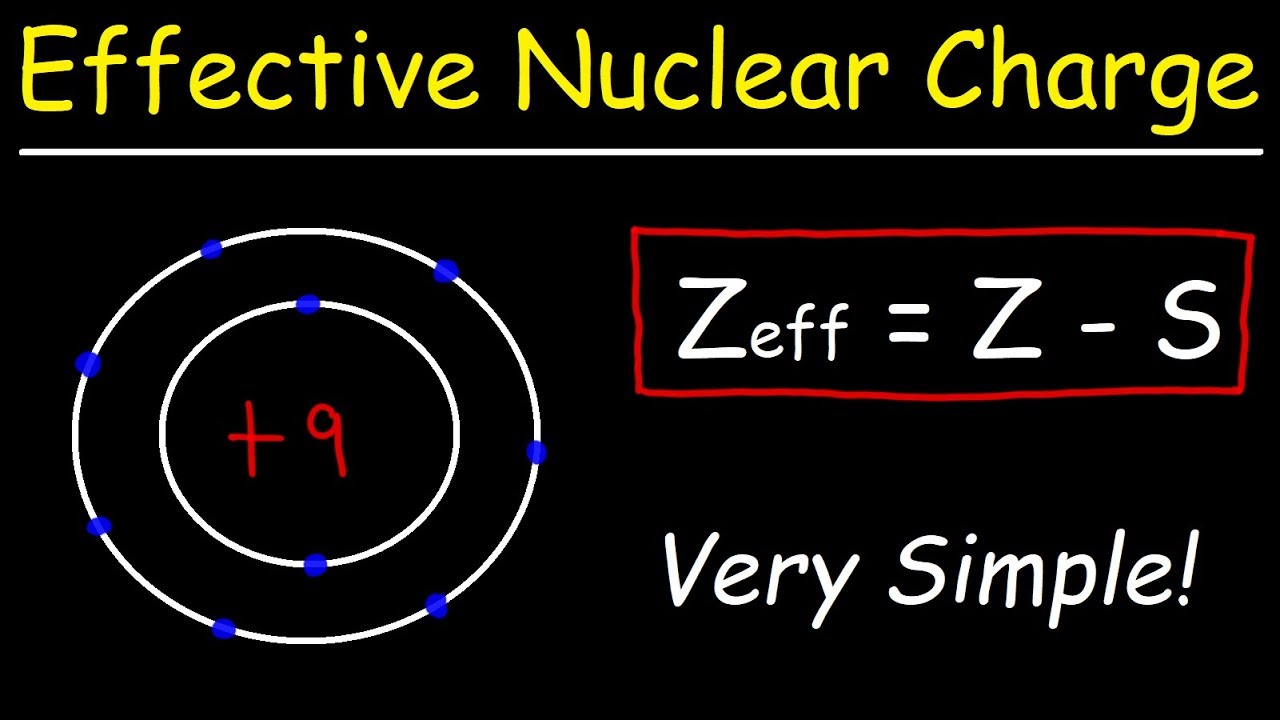
The Effective Nuclear Charge Is Determined By Subtracting From The Number Of Protons In The Nucleus (Z), The Number Of Inner Core (I.c.).
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion.