What Is The Electron Configuration For Magnesium
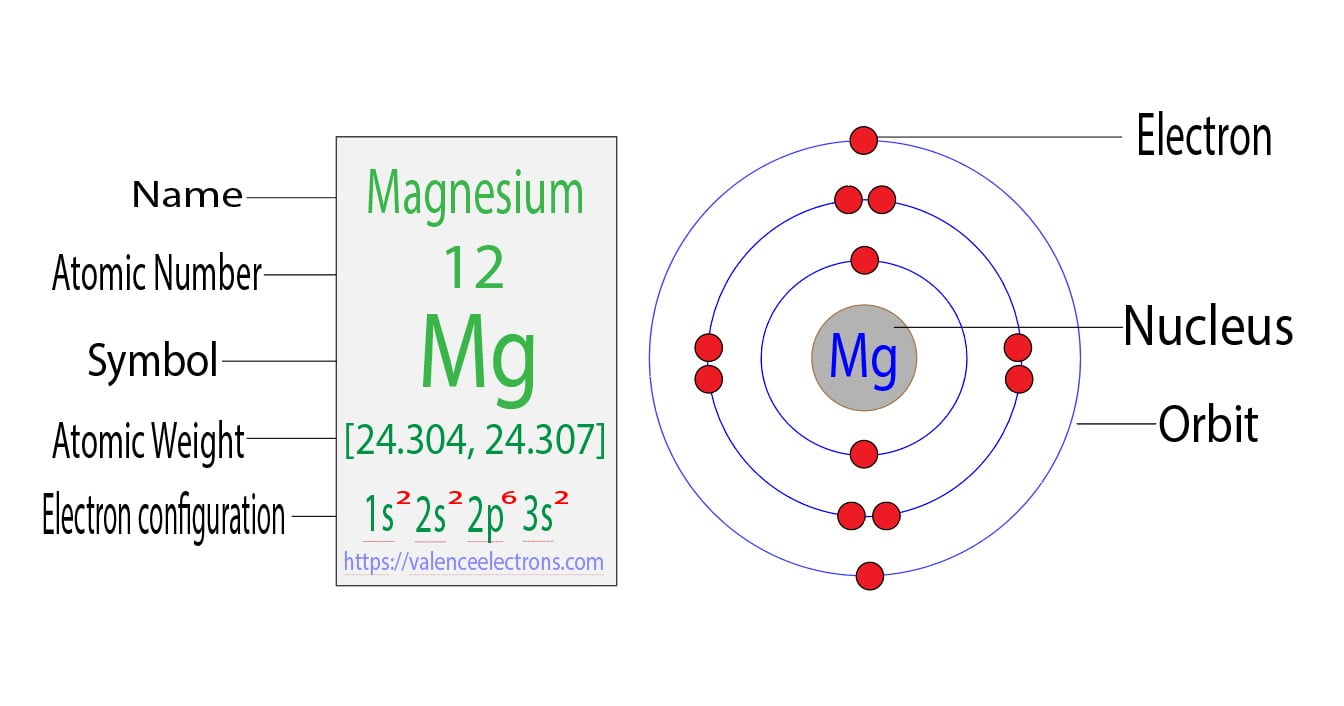
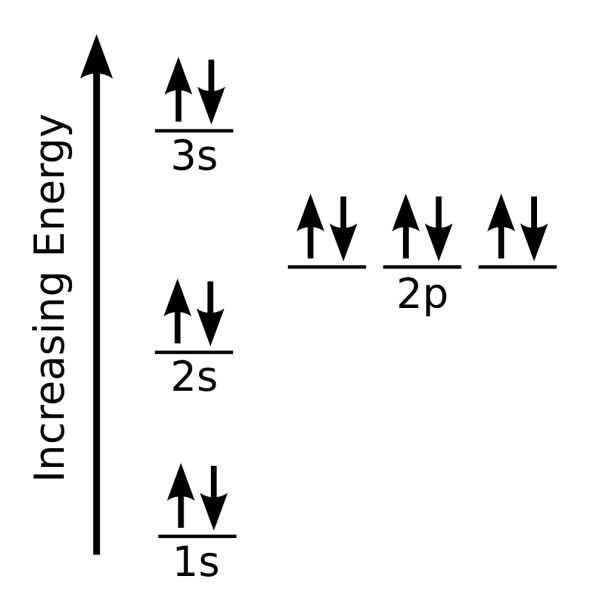
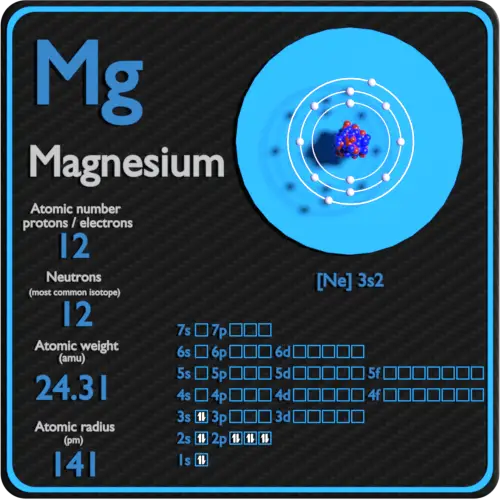
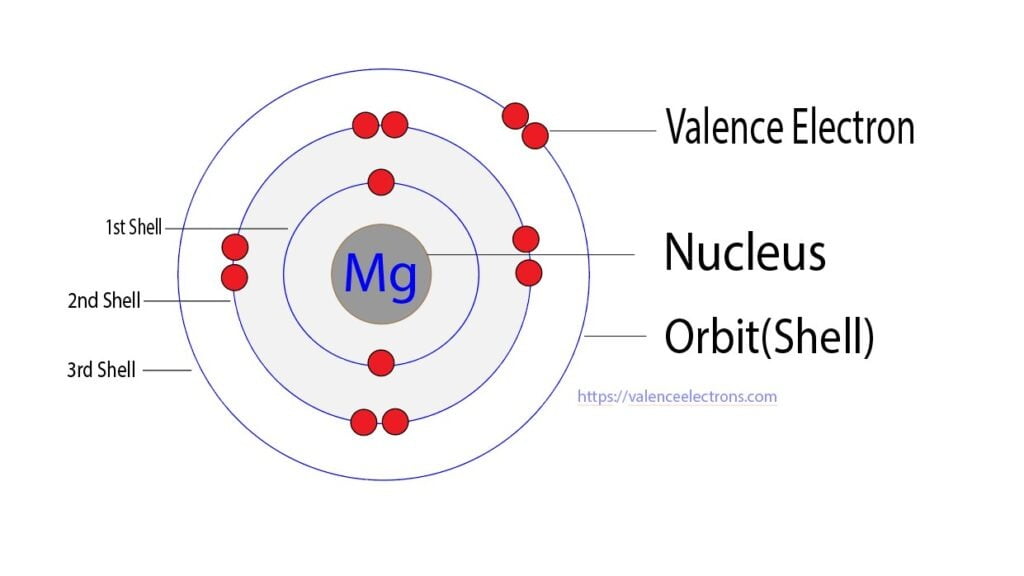
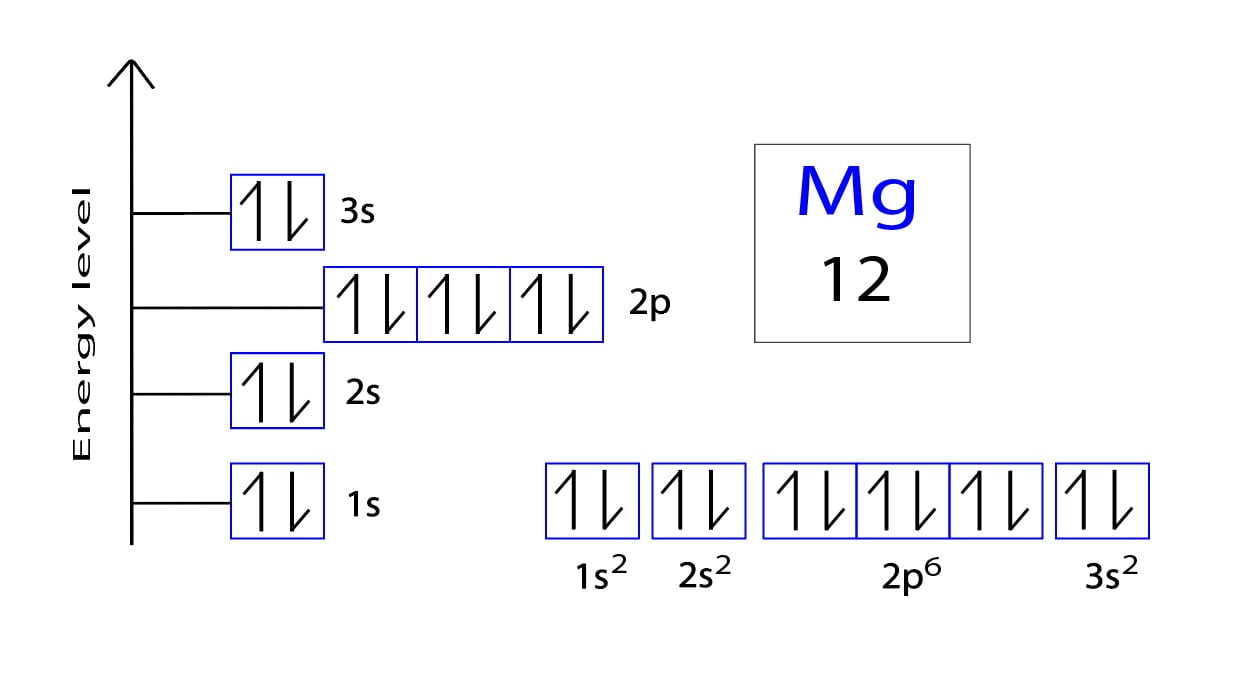
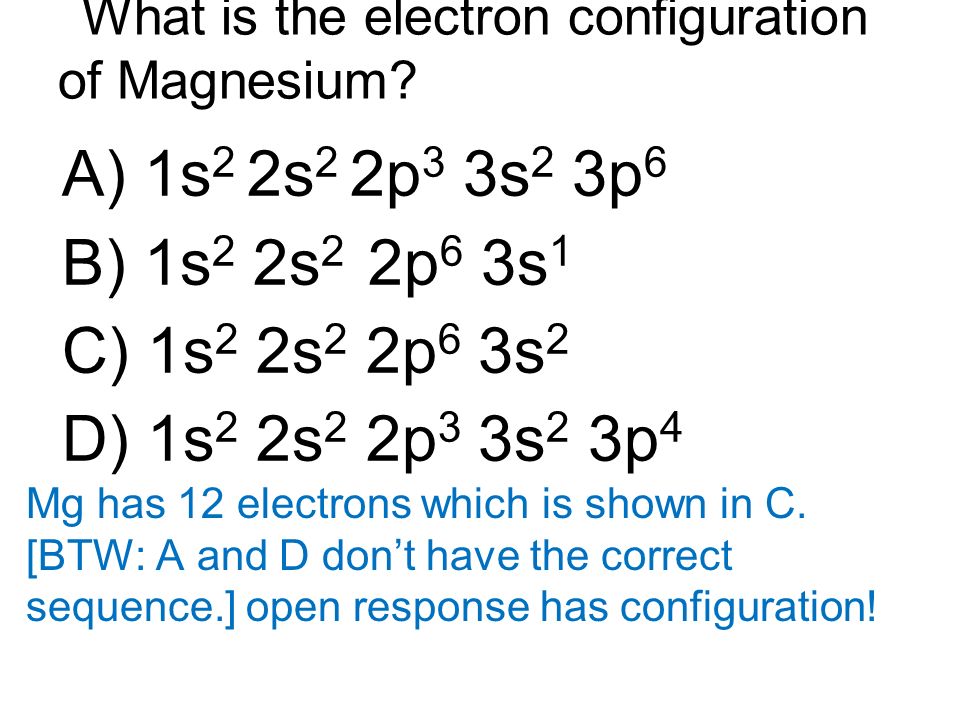
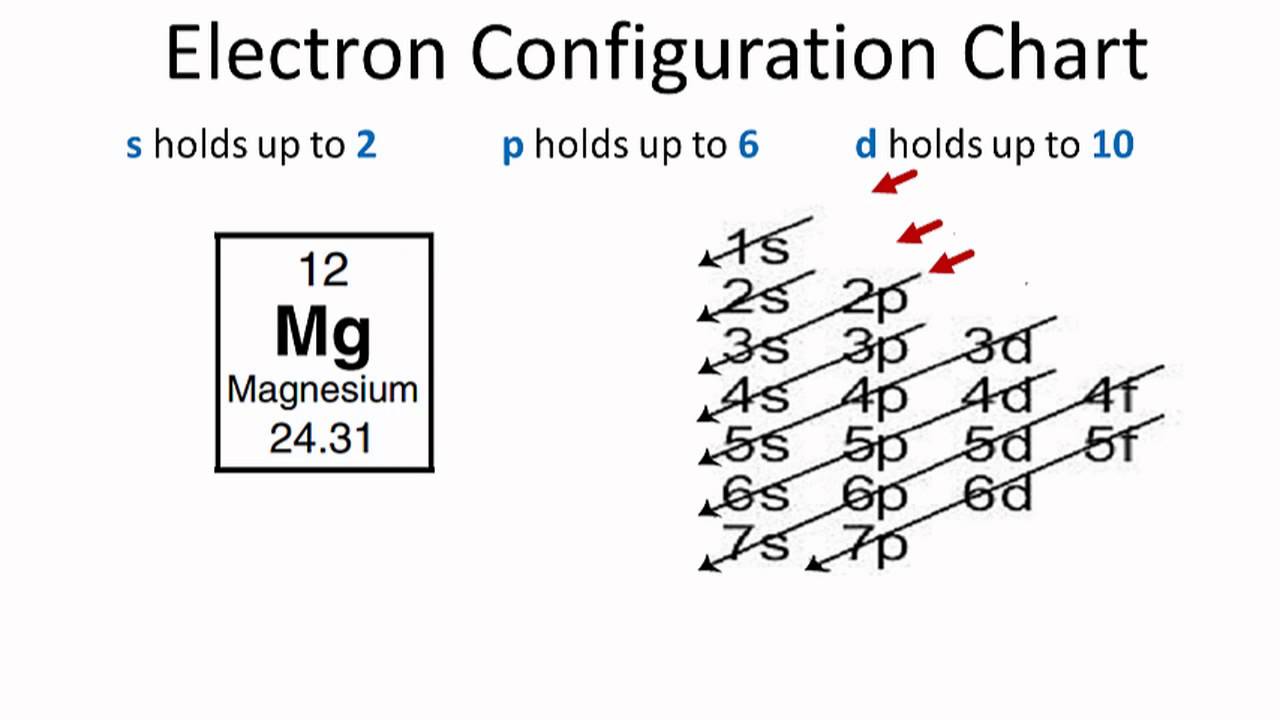
What Is The Electron Configuration For Magnesium - The shorthand electron configuration (or noble gas. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Magnesium's 12 electrons fill the 1s, 2s, 2p, and 3s orbitals. Electrons fill orbitals starting from the lowest energy level. The electron configuration of an atom. The electron configuration for neutral magnesium (mg) is 1s22s22p63s2. The electron configuration for magnesium (mg) is: The ion, mg2+, has two electrons fewer, so the outer. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s².
Magnesium's 12 electrons fill the 1s, 2s, 2p, and 3s orbitals. The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below. Electrons fill orbitals starting from the lowest energy level. The ion, mg2+, has two electrons fewer, so the outer. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Magnesium, with an atomic number. The shorthand electron configuration (or noble gas. The electron configuration of an atom. The electron configuration for magnesium (mg) is:
The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s². The shorthand electron configuration (or noble gas. The ion, mg2+, has two electrons fewer, so the outer. Magnesium, with an atomic number. The electron configuration of an atom. Electrons fill orbitals starting from the lowest energy level. The electron configuration for magnesium (mg) is: The electron configuration for neutral magnesium (mg) is 1s22s22p63s2. Electron configuration chart of all elements is mentioned in the table below. Magnesium's 12 electrons fill the 1s, 2s, 2p, and 3s orbitals.
How to Write the Electron Configuration for Magnesium (Mg)
Electrons fill orbitals starting from the lowest energy level. The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s². The electron configuration for magnesium (mg) is: The shorthand electron configuration (or noble gas. Magnesium, with an atomic number.
Magnesium Electron Configuration (Mg) with Orbital Diagram
Magnesium, with an atomic number. The electron configuration for neutral magnesium (mg) is 1s22s22p63s2. Electrons fill orbitals starting from the lowest energy level. The shorthand electron configuration (or noble gas. The ion, mg2+, has two electrons fewer, so the outer.
File Electron Configuration Magnesium Svg Best Diagram Collection
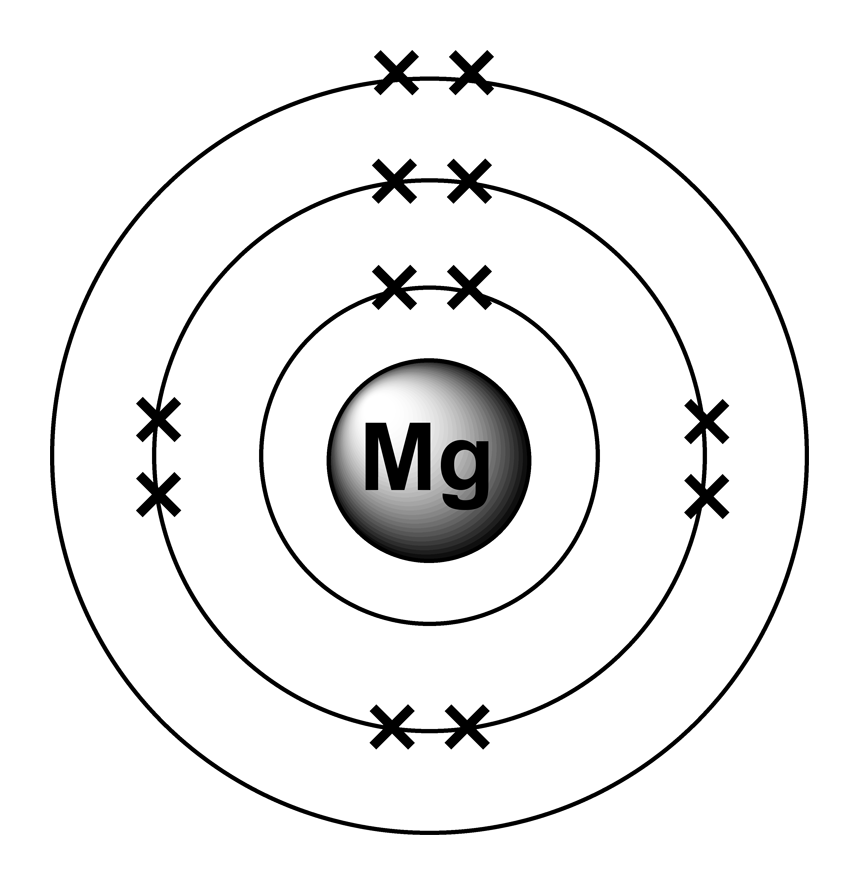
Magnesium, with an atomic number. The electron configuration of an atom. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Magnesium's 12 electrons fill the 1s, 2s, 2p, and 3s orbitals. The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s².
Magnesium Protons Neutrons Electrons Electron Configuration
Magnesium, with an atomic number. The electron configuration of an atom. The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below. The electron configuration for neutral magnesium (mg) is 1s22s22p63s2.
How to Write the Electron Configuration for Magnesium (Mg)
The shorthand electron configuration (or noble gas. The electron configuration for magnesium (mg) is: Magnesium's 12 electrons fill the 1s, 2s, 2p, and 3s orbitals. The ion, mg2+, has two electrons fewer, so the outer. The electron configuration of an atom.
Electron Configuration for Magnesium(Mg, Mg2+ ion)
The electron configuration of an atom. The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s². The electron configuration for neutral magnesium (mg) is 1s22s22p63s2. Electrons fill orbitals starting from the lowest energy level. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom.
Magnesium Electron Configuration (Mg) with Orbital Diagram
The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s². Magnesium's 12 electrons fill the 1s, 2s, 2p, and 3s orbitals. Magnesium, with an atomic number. Electrons fill orbitals starting from the lowest energy level. The electron configuration for magnesium (mg) is:
Electron arrangements
Magnesium's 12 electrons fill the 1s, 2s, 2p, and 3s orbitals. The electron configuration of an atom. The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s². When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Magnesium, with an atomic number.
Magnesium Electron Configuration (Mg) with Orbital Diagram
The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s². The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. The ion, mg2+, has two electrons fewer, so the outer. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the.
Magnesium Electron Configuration (Mg) with Orbital Diagram
The electron configuration for magnesium (mg) is: When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Magnesium, with an atomic number. The shorthand electron configuration (or noble gas. The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s².
The Electron Configuration For Magnesium (Mg) Is:
The shorthand electron configuration (or noble gas. Magnesium, with an atomic number. The electron configuration of an electrically neutral atom of magnesium is 1s² 2s²2p⁶ 3s². Magnesium's 12 electrons fill the 1s, 2s, 2p, and 3s orbitals.
The Ion, Mg2+, Has Two Electrons Fewer, So The Outer.
Electron configuration chart of all elements is mentioned in the table below. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. The electron configuration for neutral magnesium (mg) is 1s22s22p63s2. The electron configuration of an atom.