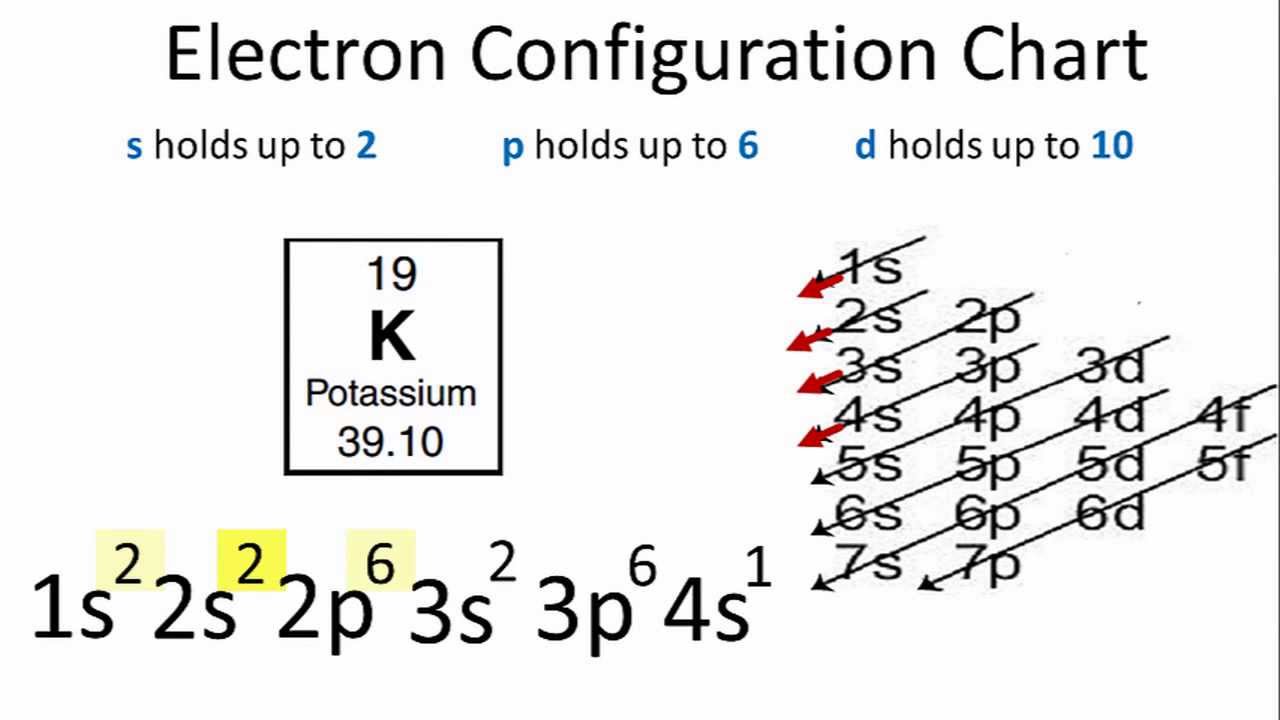
What Is The Electron Configuration For V3
What Is The Electron Configuration For V3 - The electron configuration of the v3+ ion is [ar]4s0 3d2. View rotating bohr models for all 118. Again, we remove them from the 4s orbital first,. Access detailed info on all elements: To determine the electron configuration of v3+, we remove three electrons. Atomic mass, electron configurations, charges, and more. When vanadium loses three electrons to become v 3+, the electron configuration is modified by removing three electrons from the 3d orbital. There are two unpaired electrons in v3+, making it paramagnetic.
To determine the electron configuration of v3+, we remove three electrons. There are two unpaired electrons in v3+, making it paramagnetic. Atomic mass, electron configurations, charges, and more. When vanadium loses three electrons to become v 3+, the electron configuration is modified by removing three electrons from the 3d orbital. Again, we remove them from the 4s orbital first,. Access detailed info on all elements: The electron configuration of the v3+ ion is [ar]4s0 3d2. View rotating bohr models for all 118.
The electron configuration of the v3+ ion is [ar]4s0 3d2. When vanadium loses three electrons to become v 3+, the electron configuration is modified by removing three electrons from the 3d orbital. There are two unpaired electrons in v3+, making it paramagnetic. View rotating bohr models for all 118. Again, we remove them from the 4s orbital first,. Access detailed info on all elements: To determine the electron configuration of v3+, we remove three electrons. Atomic mass, electron configurations, charges, and more.
Explanation Vanadium ion (V2+, V3+) Electron Configuration
The electron configuration of the v3+ ion is [ar]4s0 3d2. Atomic mass, electron configurations, charges, and more. Again, we remove them from the 4s orbital first,. When vanadium loses three electrons to become v 3+, the electron configuration is modified by removing three electrons from the 3d orbital. Access detailed info on all elements:
The Electron Configuration Condensed Example 1 Channels for Pearson+
There are two unpaired electrons in v3+, making it paramagnetic. When vanadium loses three electrons to become v 3+, the electron configuration is modified by removing three electrons from the 3d orbital. Access detailed info on all elements: The electron configuration of the v3+ ion is [ar]4s0 3d2. Again, we remove them from the 4s orbital first,.
😍 Electron configuration examples. Abbreviated Electron configurations
View rotating bohr models for all 118. There are two unpaired electrons in v3+, making it paramagnetic. Again, we remove them from the 4s orbital first,. Atomic mass, electron configurations, charges, and more. To determine the electron configuration of v3+, we remove three electrons.
Ground State Electron Configuration of V Periodic Table Element
Access detailed info on all elements: When vanadium loses three electrons to become v 3+, the electron configuration is modified by removing three electrons from the 3d orbital. To determine the electron configuration of v3+, we remove three electrons. The electron configuration of the v3+ ion is [ar]4s0 3d2. View rotating bohr models for all 118.
Vanadium 4+ Electron Configuration
The electron configuration of the v3+ ion is [ar]4s0 3d2. There are two unpaired electrons in v3+, making it paramagnetic. View rotating bohr models for all 118. Again, we remove them from the 4s orbital first,. To determine the electron configuration of v3+, we remove three electrons.
Box Diagram Of Electron Configuration
There are two unpaired electrons in v3+, making it paramagnetic. To determine the electron configuration of v3+, we remove three electrons. View rotating bohr models for all 118. The electron configuration of the v3+ ion is [ar]4s0 3d2. Again, we remove them from the 4s orbital first,.
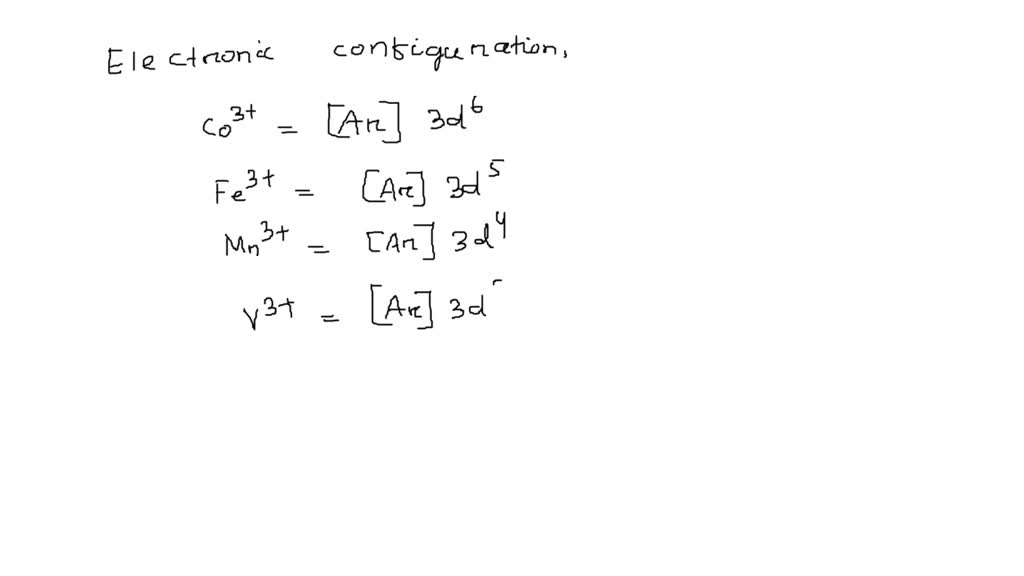
SOLVED Which 3+ ion has the ground state electron configuration [Ar
To determine the electron configuration of v3+, we remove three electrons. There are two unpaired electrons in v3+, making it paramagnetic. The electron configuration of the v3+ ion is [ar]4s0 3d2. Atomic mass, electron configurations, charges, and more. Again, we remove them from the 4s orbital first,.
Electron Configuration PPT
There are two unpaired electrons in v3+, making it paramagnetic. The electron configuration of the v3+ ion is [ar]4s0 3d2. View rotating bohr models for all 118. Access detailed info on all elements: Again, we remove them from the 4s orbital first,.
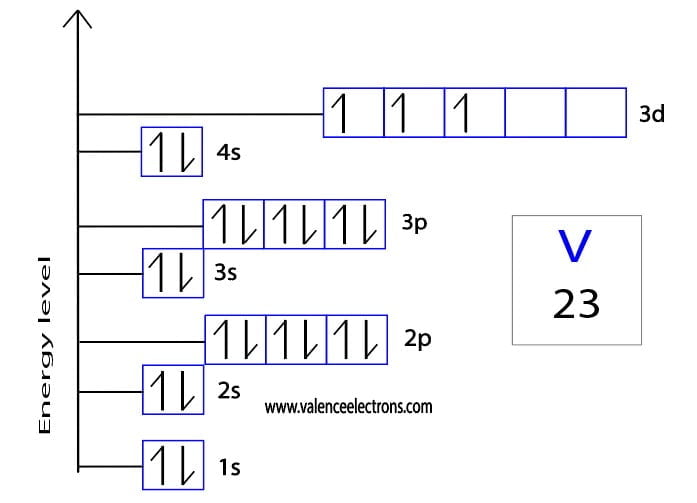
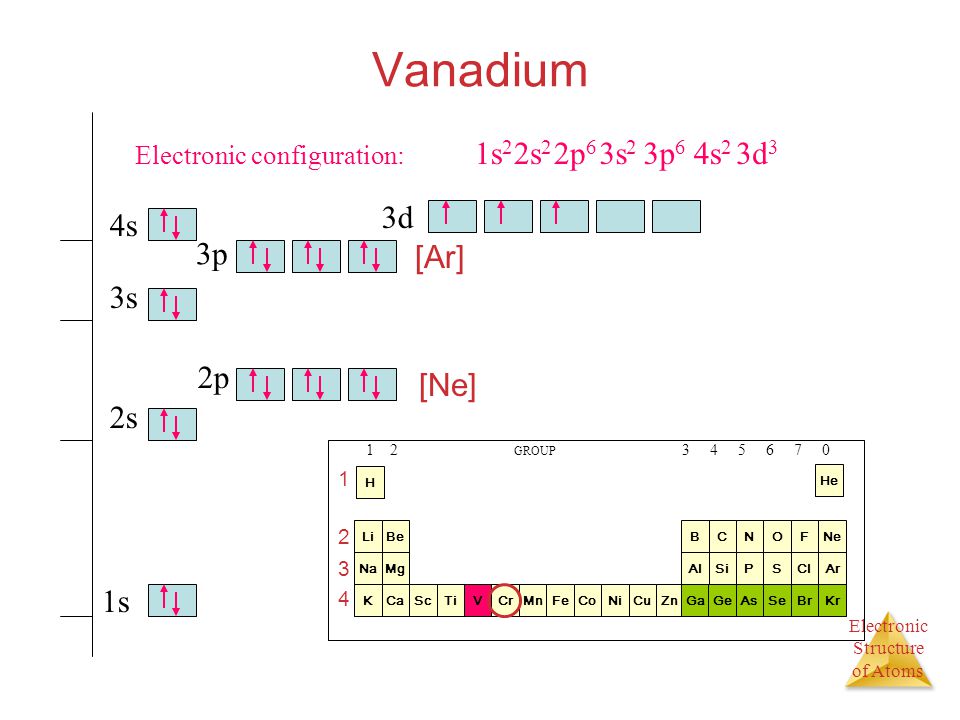
Electron Configuration of Vanadium and ions(V2+,V3+)
View rotating bohr models for all 118. Again, we remove them from the 4s orbital first,. The electron configuration of the v3+ ion is [ar]4s0 3d2. Access detailed info on all elements: When vanadium loses three electrons to become v 3+, the electron configuration is modified by removing three electrons from the 3d orbital.
Electronic+configuration_ Dynamic Periodic Table of Elements and
Access detailed info on all elements: To determine the electron configuration of v3+, we remove three electrons. Again, we remove them from the 4s orbital first,. View rotating bohr models for all 118. The electron configuration of the v3+ ion is [ar]4s0 3d2.
View Rotating Bohr Models For All 118.
Access detailed info on all elements: When vanadium loses three electrons to become v 3+, the electron configuration is modified by removing three electrons from the 3d orbital. The electron configuration of the v3+ ion is [ar]4s0 3d2. Again, we remove them from the 4s orbital first,.
There Are Two Unpaired Electrons In V3+, Making It Paramagnetic.
To determine the electron configuration of v3+, we remove three electrons. Atomic mass, electron configurations, charges, and more.