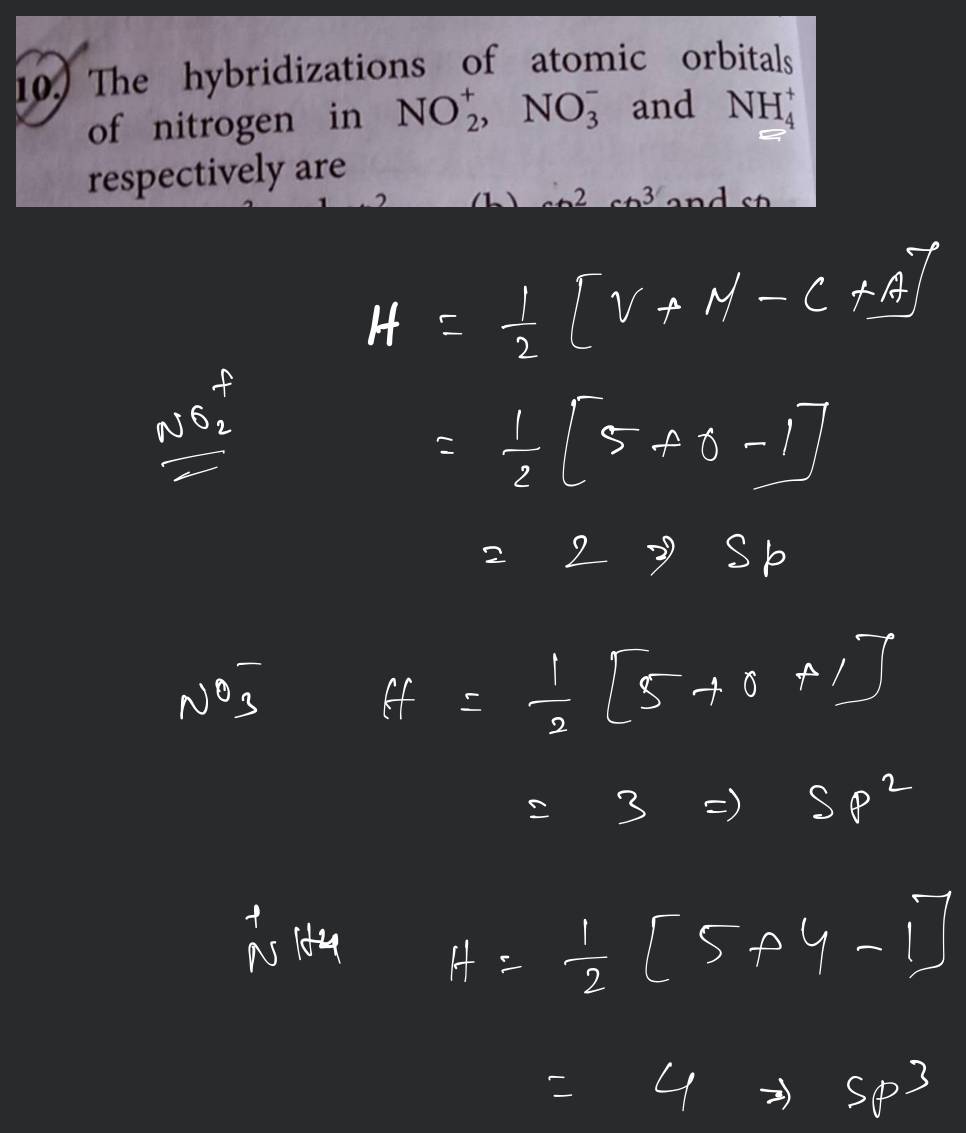What Is The Hybridization Of Nitrogen In No2
What Is The Hybridization Of Nitrogen In No2 - In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. Two of the sp 3 hybridized orbitals overlap with s orbitals from. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. As per the hybridization rule, the sum of the number of. The simple way to determine the hybridization of no 2 is by. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals.
The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The simple way to determine the hybridization of no 2 is by. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. As per the hybridization rule, the sum of the number of. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron.
As per the hybridization rule, the sum of the number of. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. The simple way to determine the hybridization of no 2 is by.
SOLVED What is the hybridization of nitrogen in the nitrite ion
As per the hybridization rule, the sum of the number of. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The simple way to determine the hybridization of no 2 is by. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. In no 2, around.
Solved What is the hybridization of the nitrogen atom in
In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. As per the hybridization rule, the sum of the number of. The simple way to determine the hybridization of no 2 is by..
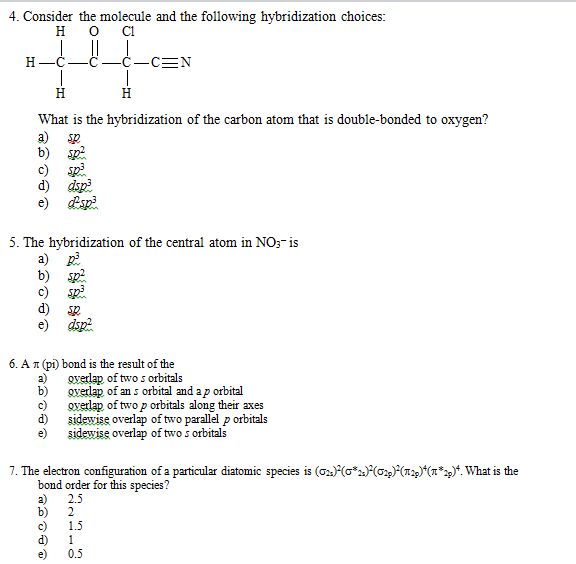
Solved 4. (6 points) Determine the hybridization of the
Two of the sp 3 hybridized orbitals overlap with s orbitals from. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. As per the hybridization rule, the sum of the number of. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. In no 2, around the nitrogen.
The hybridization of atomic orbital of nitrogen in NO2+, NO3 and NH4
The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. Two of the sp 3 hybridized orbitals overlap with s orbitals from. As per the hybridization rule, the sum of the number of..
[Solved] Identify how many N (NITROGEN) atoms have sp2
Two of the sp 3 hybridized orbitals overlap with s orbitals from. The simple way to determine the hybridization of no 2 is by. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions.
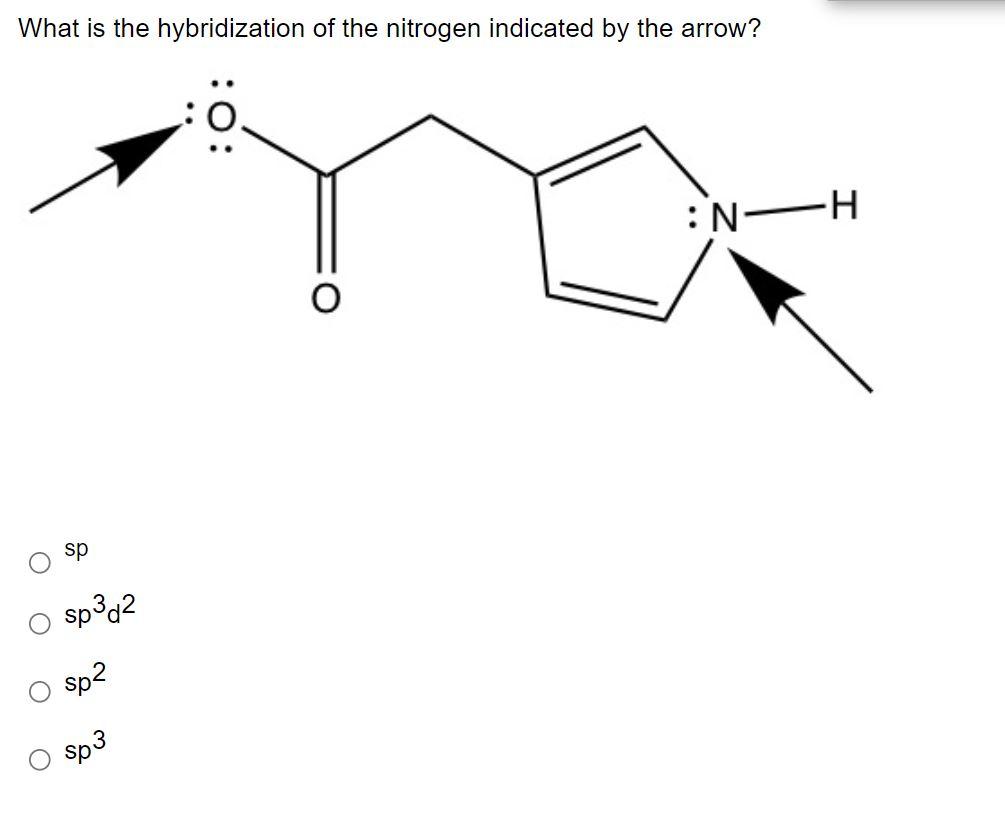
Solved What is the hybridization of the nitrogen indicated
The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. As per the hybridization rule, the sum of the number of. In no 2, around the nitrogen atom, there are two sigma bonds.
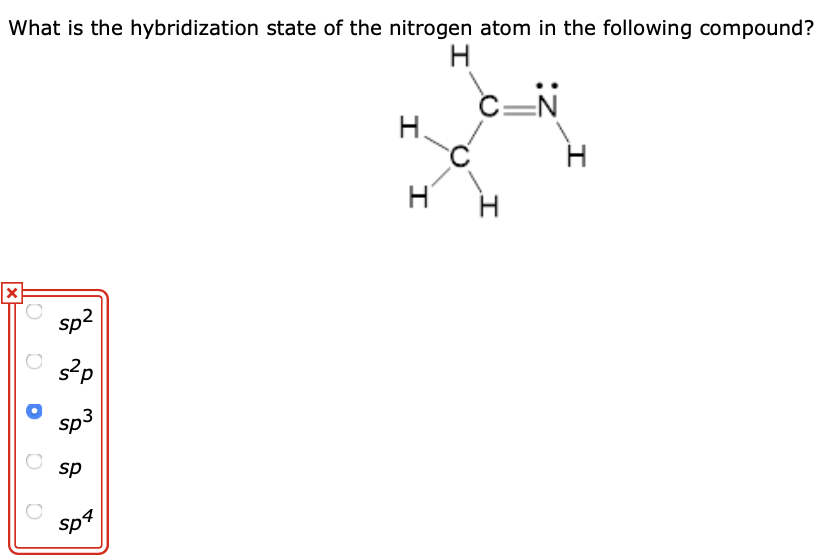
Solved What is the hybridization state of the nitrogen atom
Nitrogen dioxide (no 2) involves an sp 2 hybridization type. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. The hybridization of nitrogen varies from.
Solved 1. The hybridization of the nitrogen atom inthe
Nitrogen dioxide (no 2) involves an sp 2 hybridization type. The simple way to determine the hybridization of no 2 is by. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. In no 2, around the.
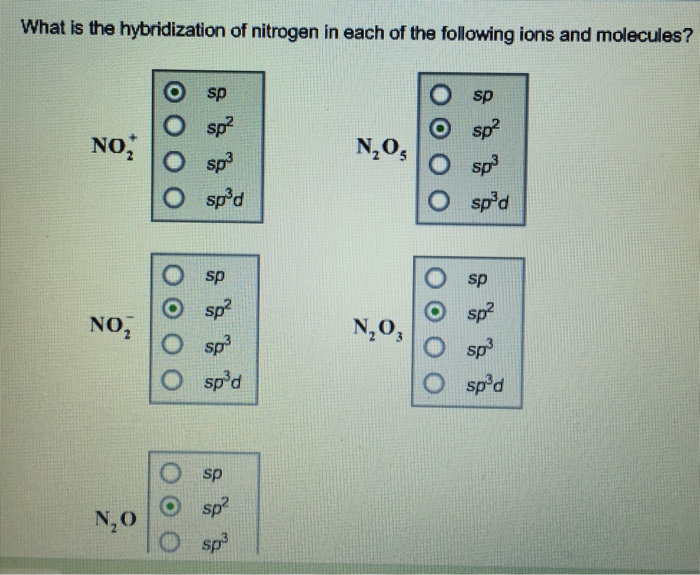
What s the hybridization of nitrogen in each of the
Nitrogen dioxide (no 2) involves an sp 2 hybridization type. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The.
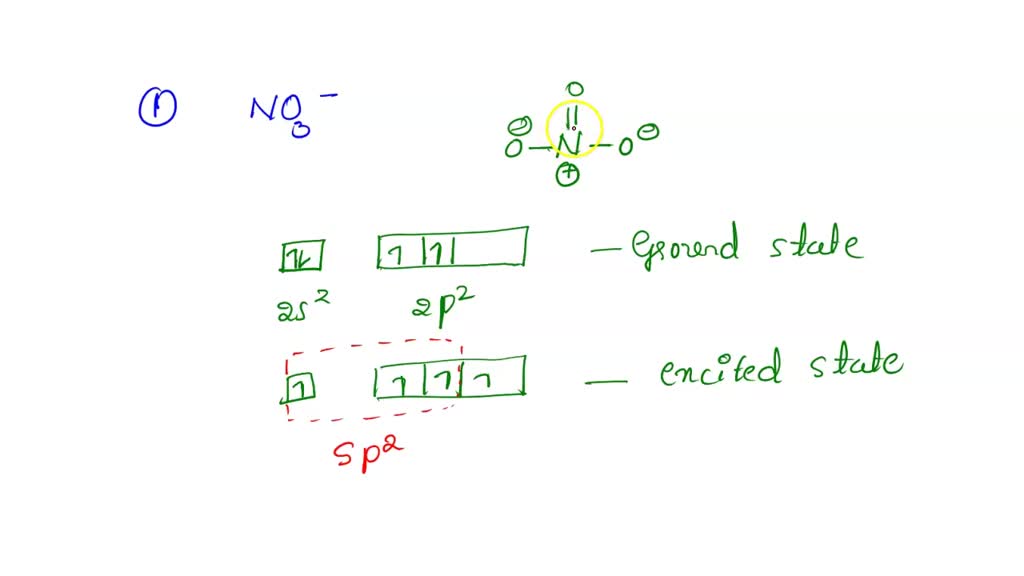
3. The hybridization of atomic orbitals of nitrogen in NO2+ ,NO2− and NH4..
Nitrogen dioxide (no 2) involves an sp 2 hybridization type. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The simple way to determine the hybridization of no 2 is by. As per the hybridization rule, the sum of the number.
Nitrogen Dioxide (No 2) Involves An Sp 2 Hybridization Type.
The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The simple way to determine the hybridization of no 2 is by.
In No 2, Around The Nitrogen Atom, There Are Two Sigma Bonds And One Unshared Electron.
As per the hybridization rule, the sum of the number of.

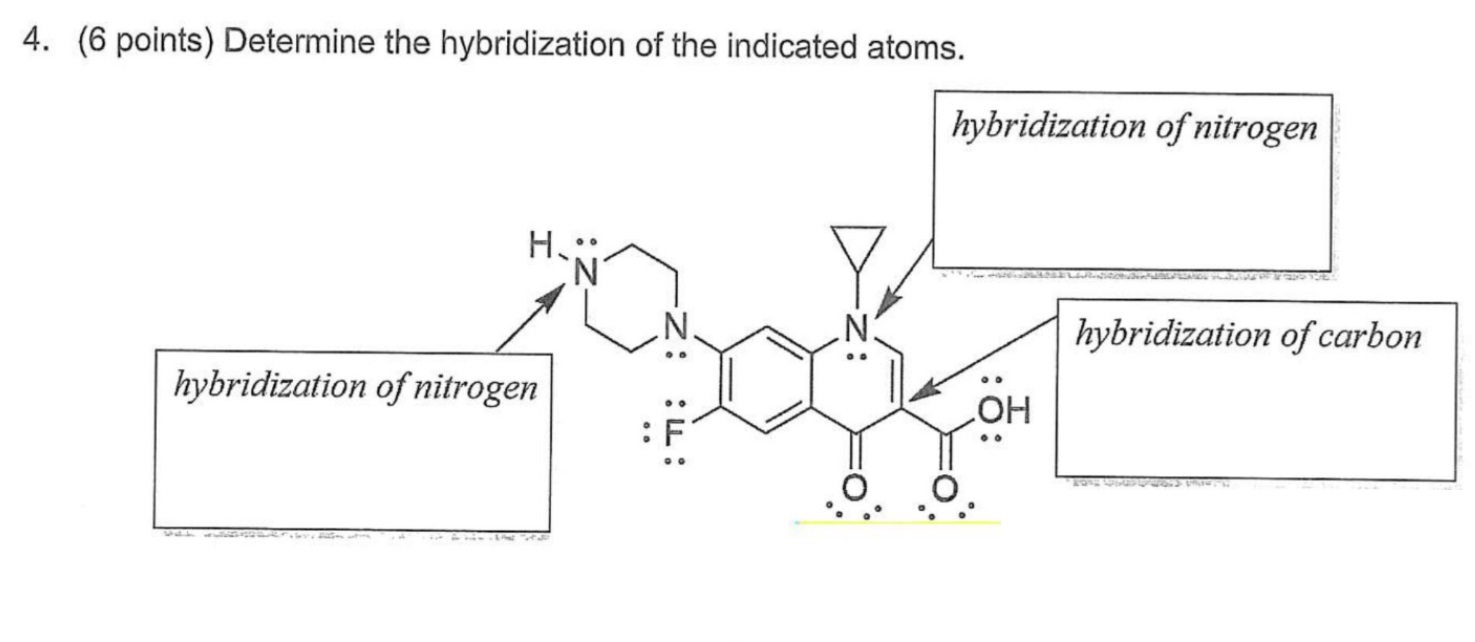

![[Solved] Identify how many N (NITROGEN) atoms have sp2](https://media.cheggcdn.com/media/250/25001471-53e2-40e1-97ef-668977575fc1/phppbkvdZ)