What Is The Hybridization Of P In Pcl3
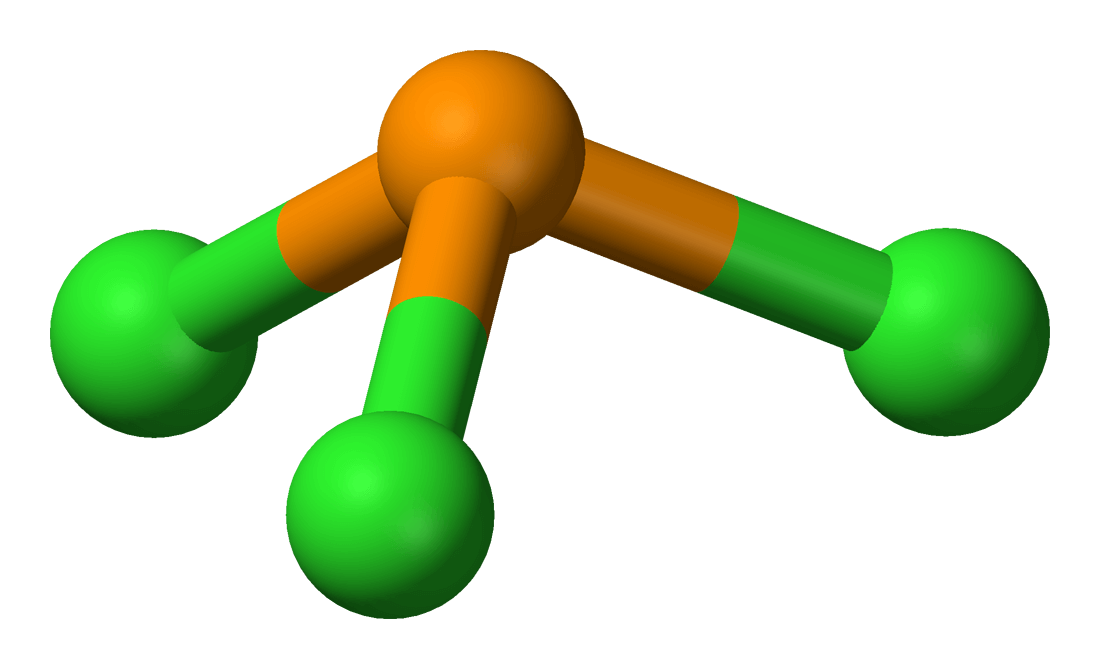
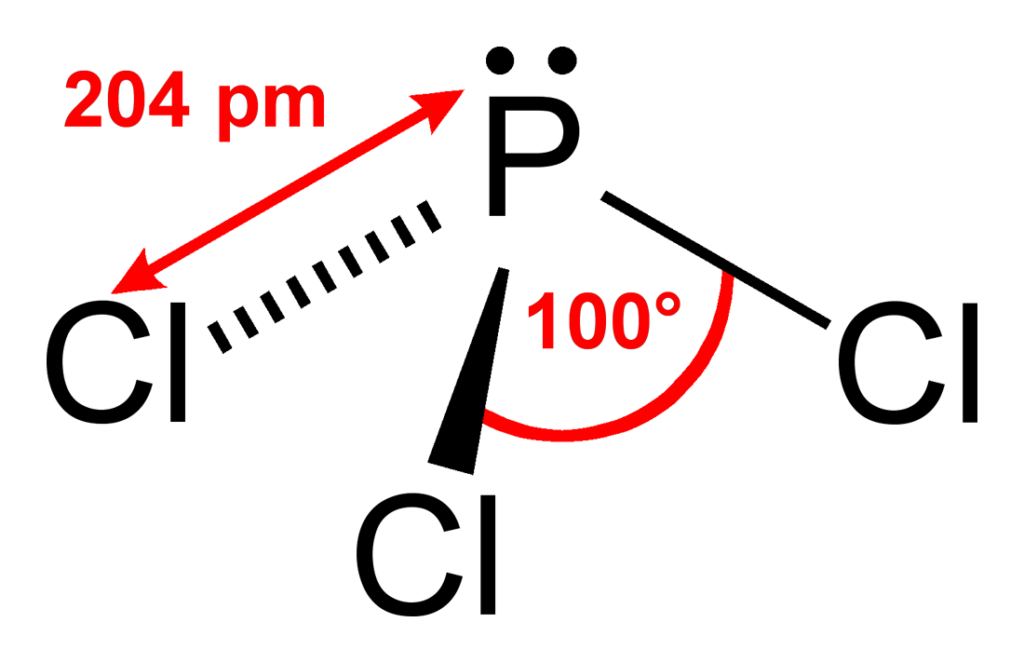
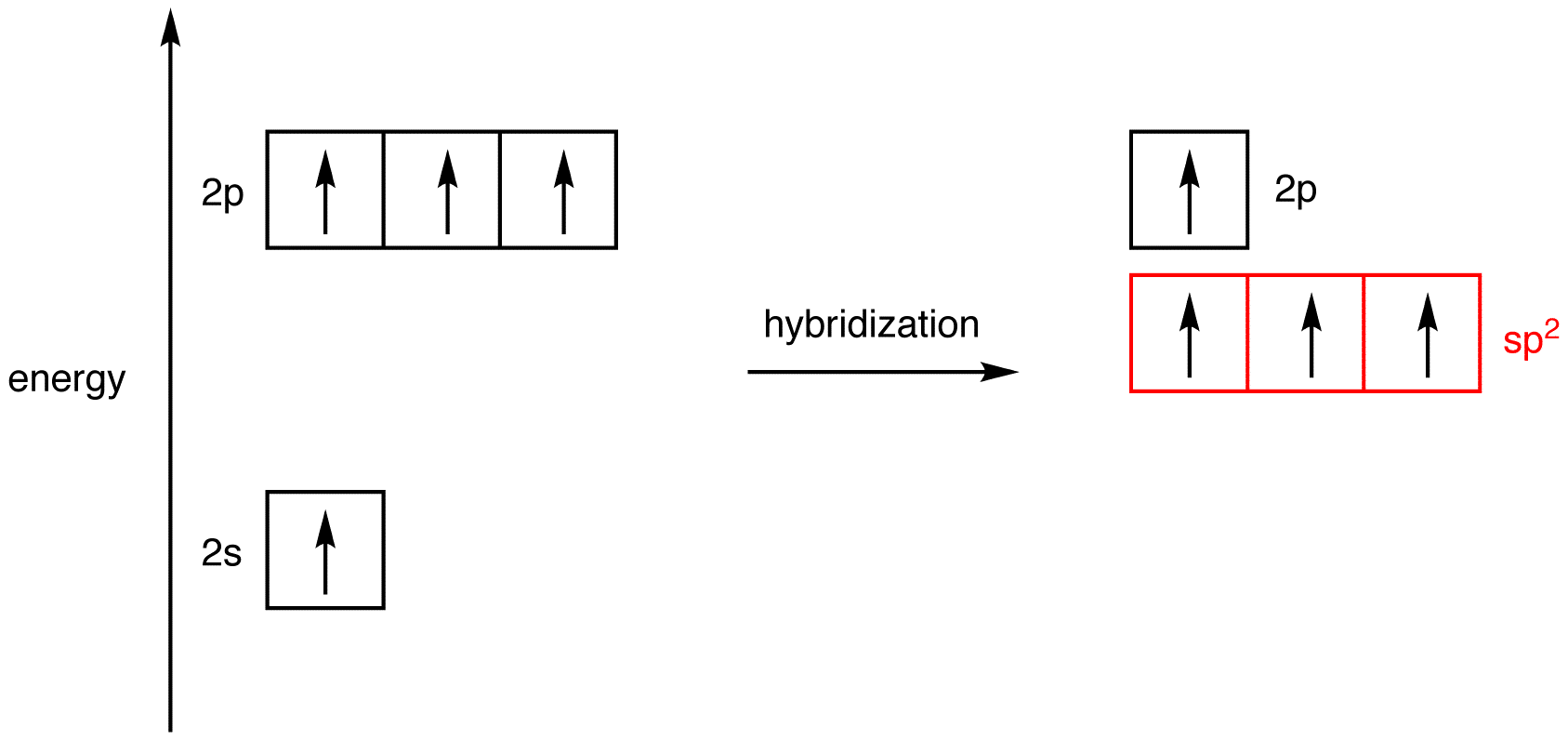
What Is The Hybridization Of P In Pcl3 - What is the hybridization of phosphorus trichloride? The hybridization of pcl3 is sp3. The hybridization of the central atom is. Phosphorus has five electrons in its outermost. The hybridization of p cl3 is sp3. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a. In the phosphorus trichloride (pcl3) molecule, the phosphorus atom is the central atom. The molecule's structure and bonding can be elucidated by understanding the concept of hybridization in the phosphorus.
What is the hybridization of phosphorus trichloride? The hybridization of p cl3 is sp3. Phosphorus has five electrons in its outermost. The molecule's structure and bonding can be elucidated by understanding the concept of hybridization in the phosphorus. The hybridization of pcl3 is sp3. In the phosphorus trichloride (pcl3) molecule, the phosphorus atom is the central atom. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a. The hybridization of the central atom is.
What is the hybridization of phosphorus trichloride? The molecule's structure and bonding can be elucidated by understanding the concept of hybridization in the phosphorus. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a. Phosphorus has five electrons in its outermost. In the phosphorus trichloride (pcl3) molecule, the phosphorus atom is the central atom. The hybridization of p cl3 is sp3. The hybridization of the central atom is. The hybridization of pcl3 is sp3.
PCL3 Molecular Electron Geometry, Lewis Structure, Bond Angles and
The hybridization of p cl3 is sp3. In the phosphorus trichloride (pcl3) molecule, the phosphorus atom is the central atom. The hybridization of pcl3 is sp3. The hybridization of the central atom is. Phosphorus has five electrons in its outermost.
Hybridization Chart
In the phosphorus trichloride (pcl3) molecule, the phosphorus atom is the central atom. What is the hybridization of phosphorus trichloride? The hybridization of the central atom is. Phosphorus has five electrons in its outermost. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a.
PCl3 Lewis Structure, Molecular Geometry, Bond Angle,, 43 OFF
Phosphorus has five electrons in its outermost. What is the hybridization of phosphorus trichloride? The hybridization of the central atom is. The hybridization of p cl3 is sp3. The hybridization of pcl3 is sp3.
What is the shape and hybridization of the PCl3 molecule?
The hybridization of the central atom is. The hybridization of p cl3 is sp3. In the phosphorus trichloride (pcl3) molecule, the phosphorus atom is the central atom. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a. What is the hybridization of phosphorus trichloride?
P4s3 hybridization
What is the hybridization of phosphorus trichloride? The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a. The hybridization of p cl3 is sp3. The hybridization of pcl3 is sp3. Phosphorus has five electrons in its outermost.
PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and
Phosphorus has five electrons in its outermost. In the phosphorus trichloride (pcl3) molecule, the phosphorus atom is the central atom. The hybridization of the central atom is. The molecule's structure and bonding can be elucidated by understanding the concept of hybridization in the phosphorus. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a.
PCL3 Molecular Electron Geometry, Lewis Structure, Bond Angles and
Phosphorus has five electrons in its outermost. The hybridization of p cl3 is sp3. The hybridization of pcl3 is sp3. The molecule's structure and bonding can be elucidated by understanding the concept of hybridization in the phosphorus. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a.
What is the hybridization of each carbon in this molecule? Socratic
The hybridization of the central atom is. What is the hybridization of phosphorus trichloride? In the phosphorus trichloride (pcl3) molecule, the phosphorus atom is the central atom. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a. The hybridization of p cl3 is sp3.
PCl3 Lewis Structure, Molecular Geometry, Bond Angle,, 43 OFF
The hybridization of the central atom is. The hybridization of pcl3 is sp3. What is the hybridization of phosphorus trichloride? Phosphorus has five electrons in its outermost. The hybridization of p cl3 is sp3.
The central atom assumes sp3hybridization in (a) PCl3 (c) BF3 (b) SO..
The hybridization of pcl3 is sp3. What is the hybridization of phosphorus trichloride? The hybridization of p cl3 is sp3. Phosphorus has five electrons in its outermost. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a.
The Hybridization Of Pcl3 Is Sp3.
Phosphorus has five electrons in its outermost. The hybridization of the central atom is. The hybridization of a molecule generally gives us ideas about the mixing of orbitals in a. The hybridization of p cl3 is sp3.
In The Phosphorus Trichloride (Pcl3) Molecule, The Phosphorus Atom Is The Central Atom.
The molecule's structure and bonding can be elucidated by understanding the concept of hybridization in the phosphorus. What is the hybridization of phosphorus trichloride?