What Is The Hybridization Of The Central Atom In Sicl4
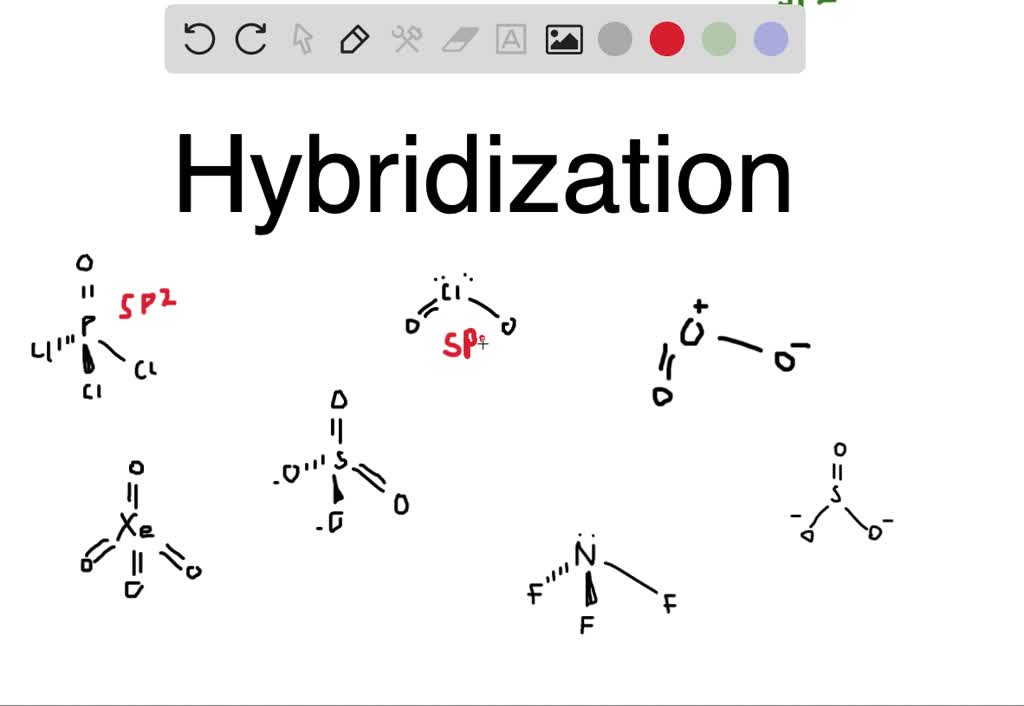
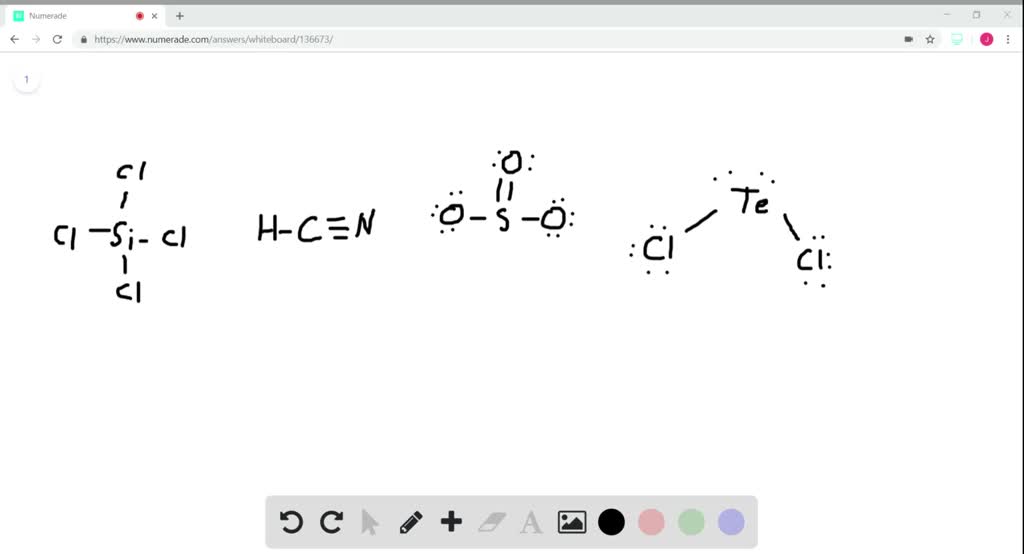
What Is The Hybridization Of The Central Atom In Sicl4 - This is determined by the number of electron groups. In the molecule sicl4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. The central atom is c (carbon), surrounded by h (hydrogen) and n (nitrogen) atoms. The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized. Thus, the hybridization of the central atom (si) in sicl4 is sp³. The central atom in sicl₄ (silicon tetrachloride) is silicon and its hybridization is sp3. This means that the silicon atom has one s orbital. Here are the hybridizations for each compound listed: In the molecule s i c l 4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the.
The central atom is c (carbon), surrounded by h (hydrogen) and n (nitrogen) atoms. This means that the silicon atom has one s orbital. The central atom in sicl₄ (silicon tetrachloride) is silicon and its hybridization is sp3. Here are the hybridizations for each compound listed: The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized. This is determined by the number of electron groups. In the molecule sicl4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. In the molecule s i c l 4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. Thus, the hybridization of the central atom (si) in sicl4 is sp³.
In the molecule sicl4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. In the molecule s i c l 4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. This is determined by the number of electron groups. The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized. Here are the hybridizations for each compound listed: Thus, the hybridization of the central atom (si) in sicl4 is sp³. The central atom is c (carbon), surrounded by h (hydrogen) and n (nitrogen) atoms. The central atom in sicl₄ (silicon tetrachloride) is silicon and its hybridization is sp3. This means that the silicon atom has one s orbital.
SOLVED Give the expected hybridization of the central atom for the
Here are the hybridizations for each compound listed: This means that the silicon atom has one s orbital. In the molecule sicl4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized..
SOLVED What is the hybridization of the central atom in CH4
Thus, the hybridization of the central atom (si) in sicl4 is sp³. The central atom in sicl₄ (silicon tetrachloride) is silicon and its hybridization is sp3. The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized. The central atom is c (carbon), surrounded by h (hydrogen) and n (nitrogen) atoms. In the molecule s i.
What Is the Hybridization of the Central Atom in Sicl4 RaynaancePhelps
This is determined by the number of electron groups. The central atom in sicl₄ (silicon tetrachloride) is silicon and its hybridization is sp3. This means that the silicon atom has one s orbital. In the molecule sicl4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the..
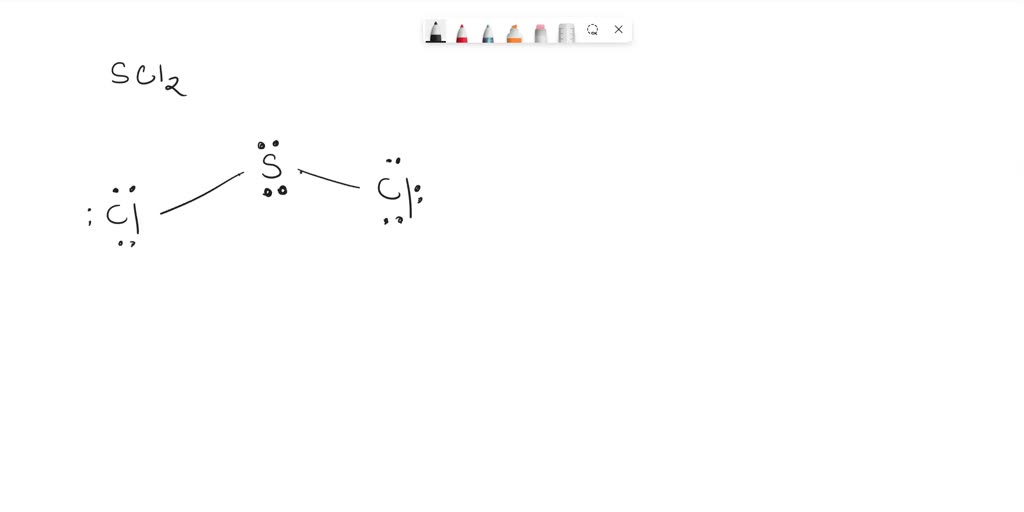
SOLVED What is the hybridization of the central atom in SCl2
The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized. Here are the hybridizations for each compound listed: The central atom is c (carbon), surrounded by h (hydrogen) and n (nitrogen) atoms. This is determined by the number of electron groups. In the molecule sicl4 the central atom si has 4 valence electrons where the.
What is the hybridization of \ce{SiCl4} central atom? Quizlet
In the molecule sicl4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized. This is determined by the number of electron groups. Here are the hybridizations for each compound listed: Thus,.
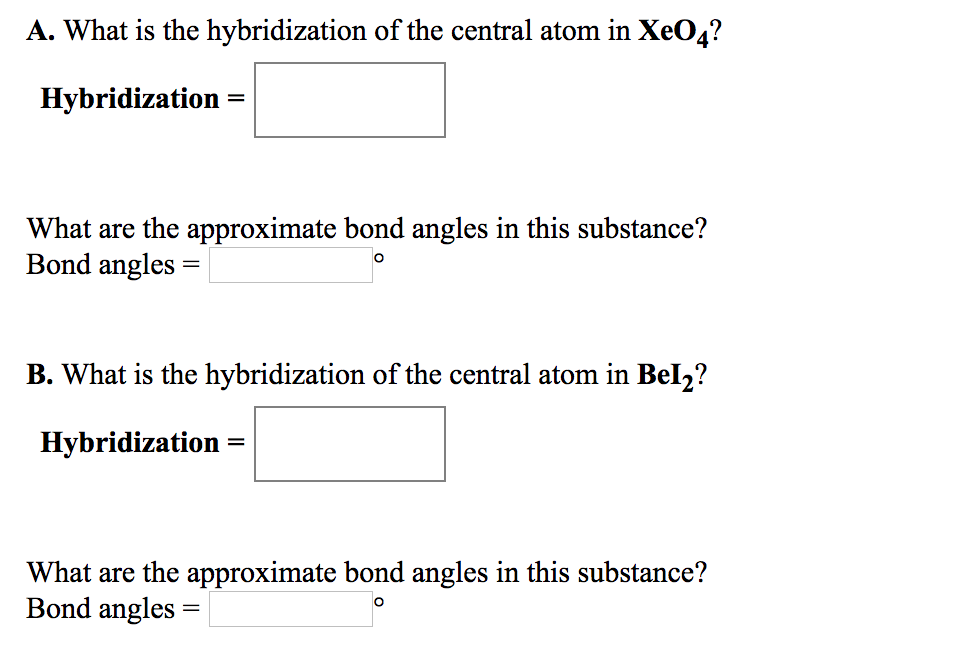
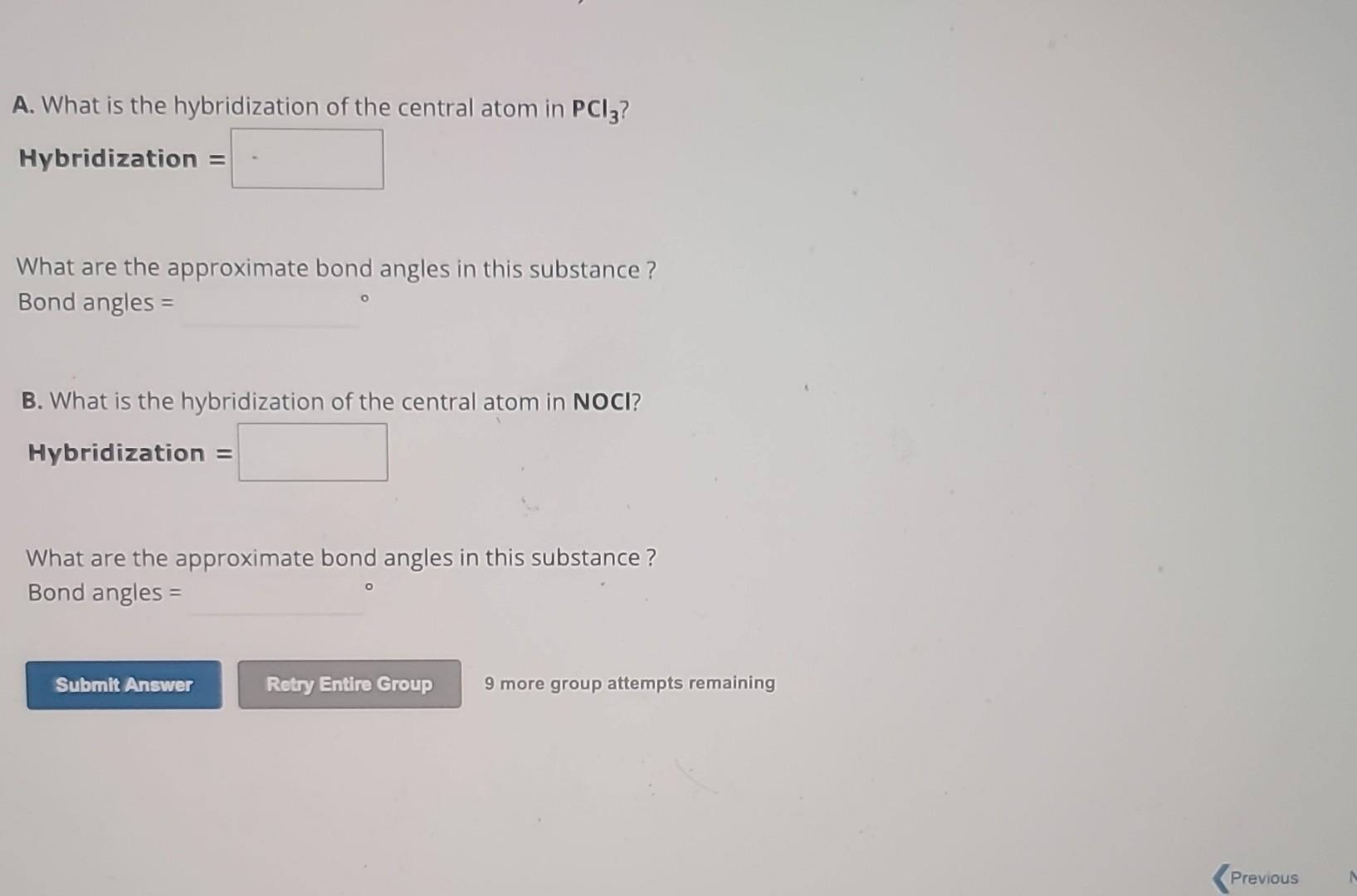
Solved A. What is the hybridization of the central atom in
The central atom is c (carbon), surrounded by h (hydrogen) and n (nitrogen) atoms. In the molecule s i c l 4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized..
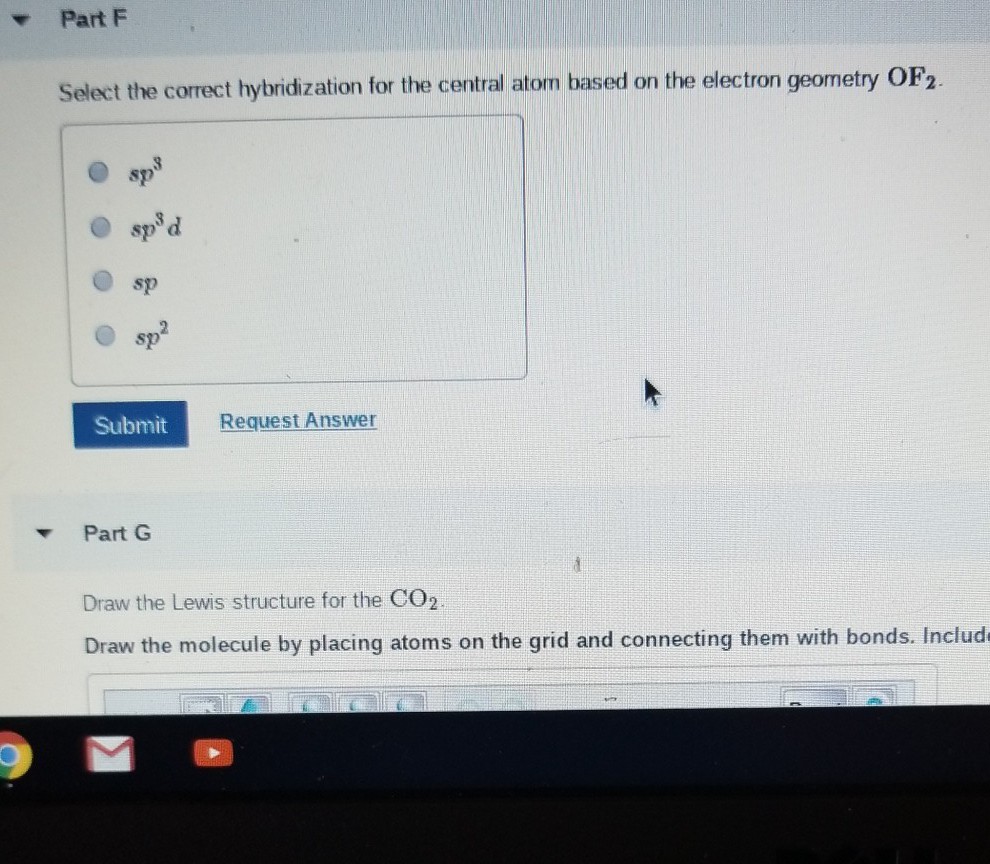
Solved Part B Select the correct hybridization for the
Here are the hybridizations for each compound listed: In the molecule sicl4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. The central atom in sicl₄ (silicon tetrachloride) is silicon and its hybridization is sp3. Thus, the hybridization of the central atom (si) in sicl4 is.
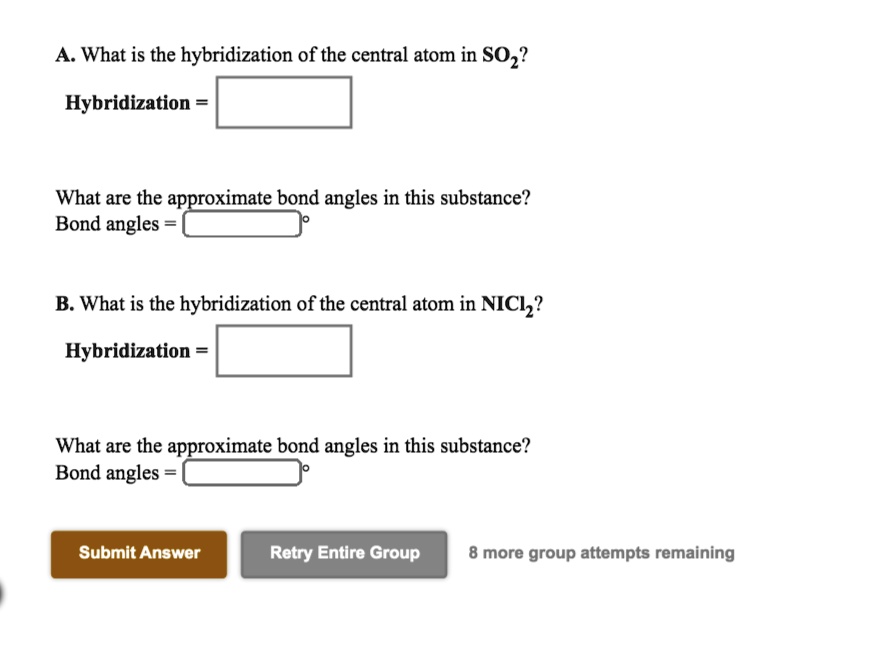
SOLVED What is the hybridization of the central atom in SOz
The central atom is c (carbon), surrounded by h (hydrogen) and n (nitrogen) atoms. This means that the silicon atom has one s orbital. In the molecule s i c l 4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. In the molecule sicl4 the.
Solved A. What is the hybridization of the central atom in
In the molecule s i c l 4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. This is determined by the number of electron groups. The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized. This means that the silicon.
SOLVED SHORT ANSWER QUESTIONS 12. For SiCl4 Draw the partial orbital
The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized. The central atom is c (carbon), surrounded by h (hydrogen) and n (nitrogen) atoms. This means that the silicon atom has one s orbital. Here are the hybridizations for each compound listed: The central atom in sicl₄ (silicon tetrachloride) is silicon and its hybridization is.
Here Are The Hybridizations For Each Compound Listed:
The central atom is c (carbon), surrounded by h (hydrogen) and n (nitrogen) atoms. In the molecule s i c l 4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the. Thus, the hybridization of the central atom (si) in sicl4 is sp³. In the molecule sicl4 the central atom si has 4 valence electrons where the si atom is forming 4 sigma bonds with cl atoms and therefore the.
This Is Determined By The Number Of Electron Groups.
The central atom in sicl₄ (silicon tetrachloride) is silicon and its hybridization is sp3. This means that the silicon atom has one s orbital. The central silicon atom in sicl4 has a tetrahedral geometry, meaning it is sp3 hybridized.