What Is The Lewis Structure For H2S
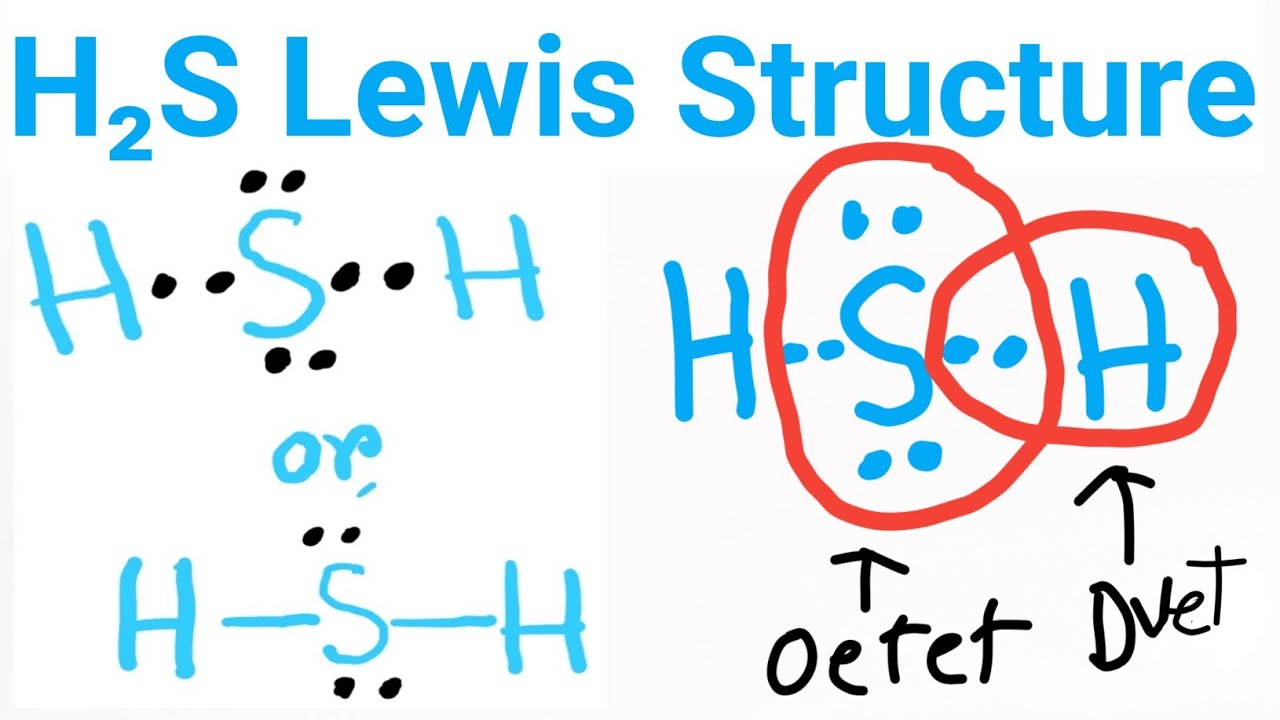
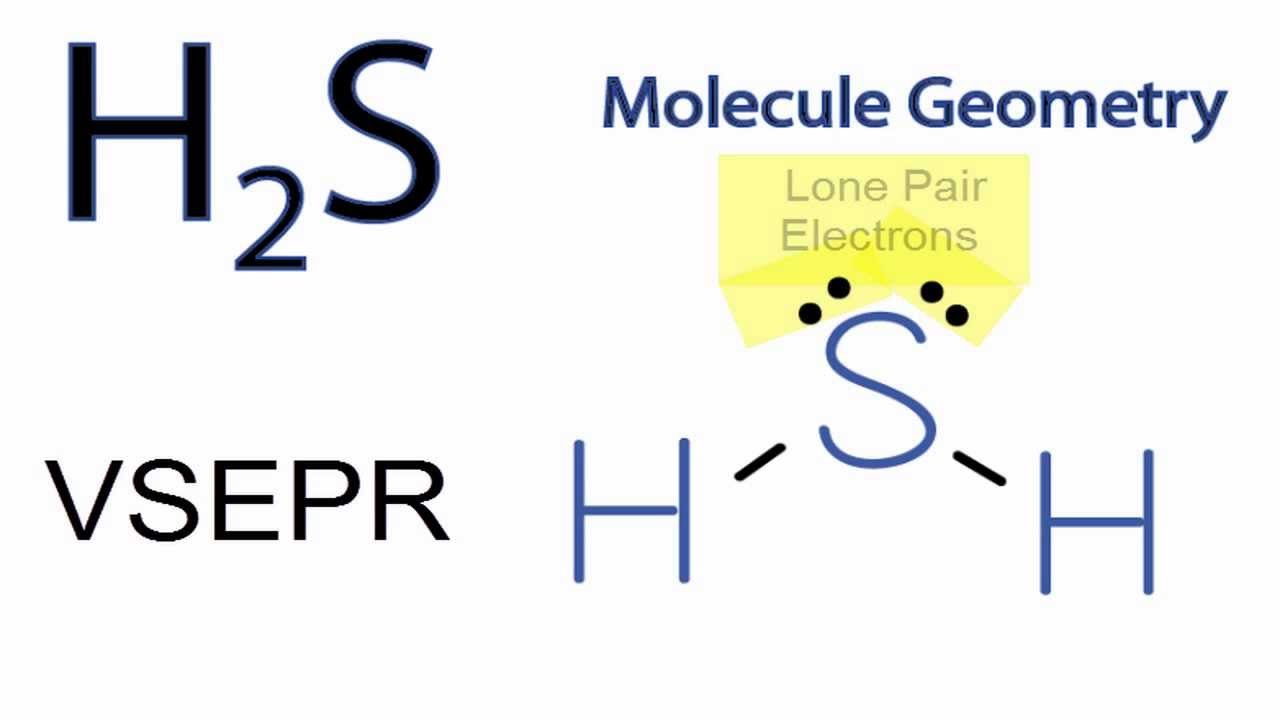
What Is The Lewis Structure For H2S - The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded. The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees.
The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees. The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded.
The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded. The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees.
H2S Lewis Structure How To Draw The Dot Structure For H2S, 43 OFF
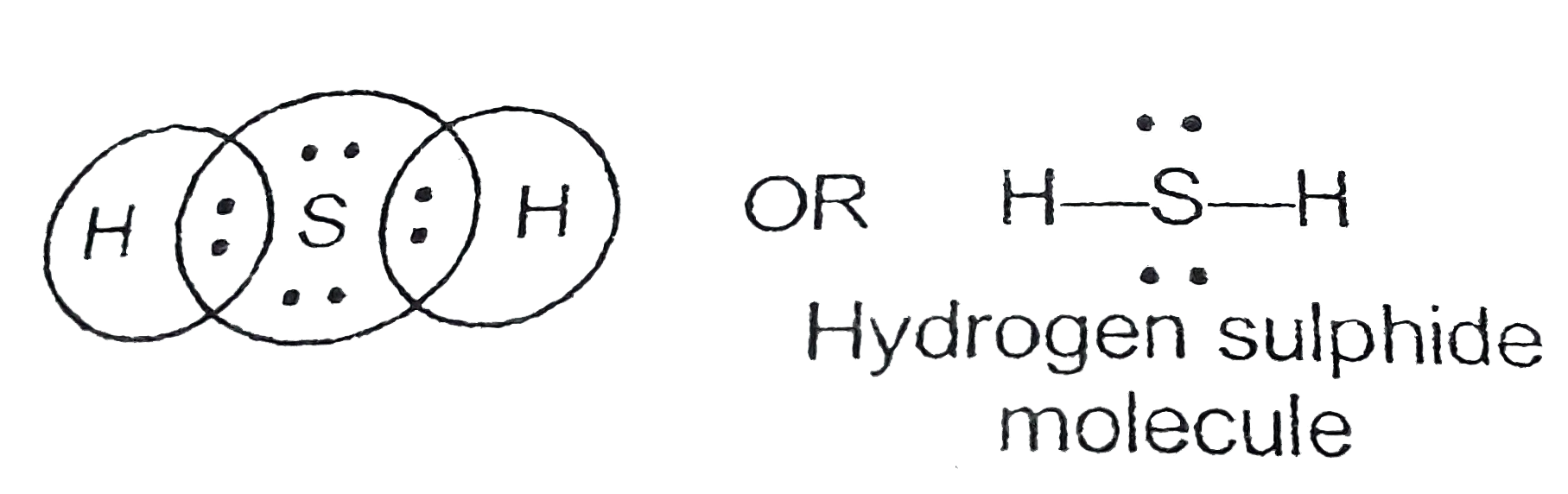
The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded. The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees.
H2S Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded. The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees.
H2S Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
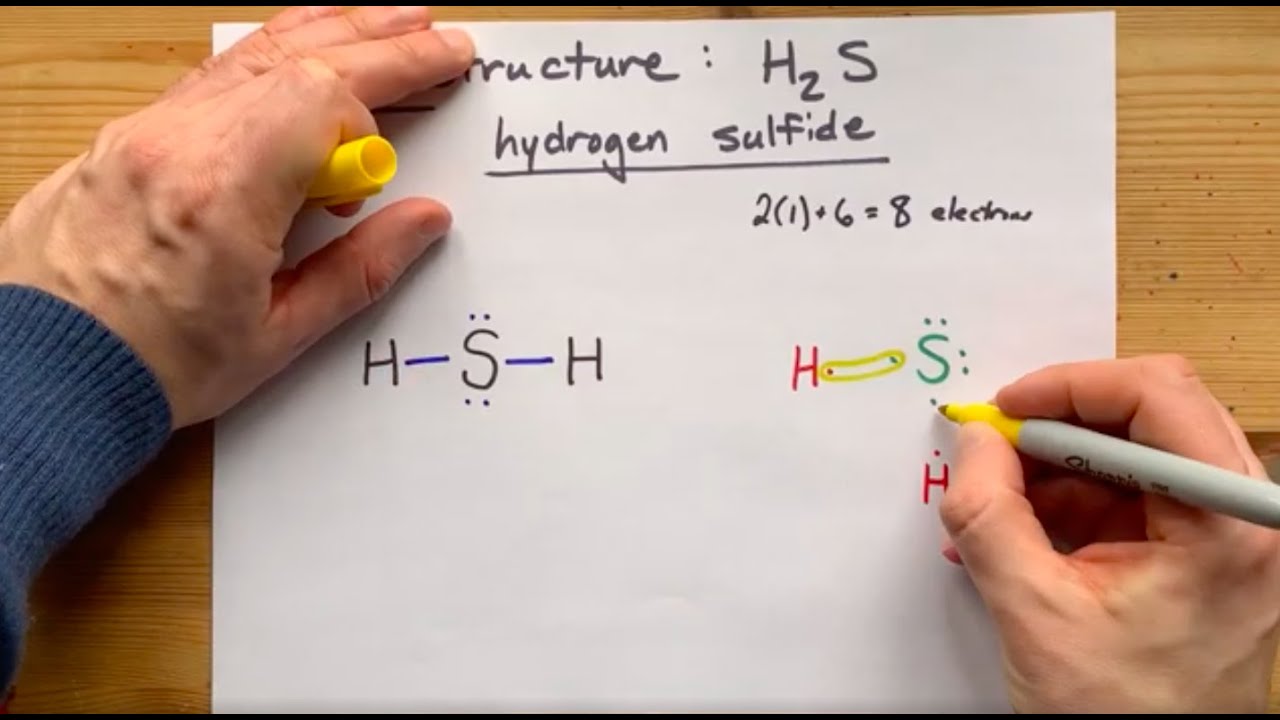
The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded. The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees.
H2S polar or nonpolar Polar, Molecular geometry, Science education
The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees. The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded.
31+ H2S Lewis Structure Pictures Bepe Enthusiastic
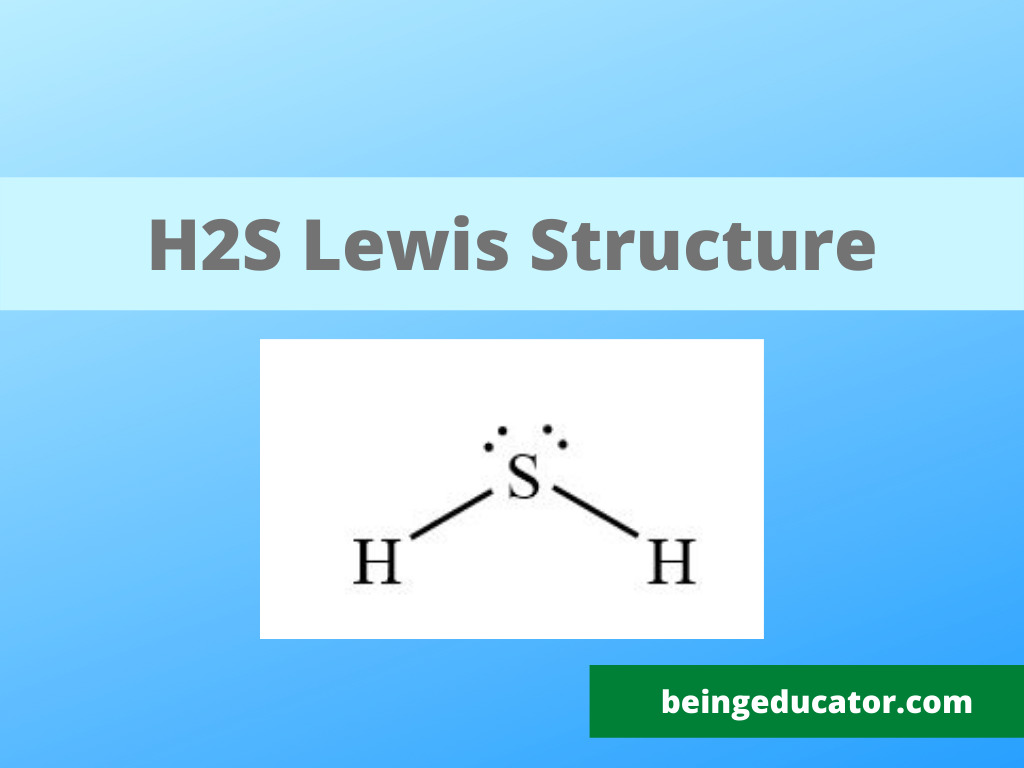
The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded. The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees.
8 Lewis Dot Structure Of H2s How To Draw Lewis
The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees. The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded.
Sh2 Lewis Structure
The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded. The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees.
H2S Lewis Structure How To Draw The Dot Structure For H2S, 46 OFF
The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees. The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded.
H2S Lewis Structure
The lewis structure of hydrogen sulfide (h2s) features a central sulfur atom with two lone pairs and two hydrogen atoms bonded. The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees.
The Lewis Structure Of Hydrogen Sulfide (H2S) Features A Central Sulfur Atom With Two Lone Pairs And Two Hydrogen Atoms Bonded.
The lewis structure of h2s suggests a bent geometry, with a bond angle of approximately 104.5 degrees.