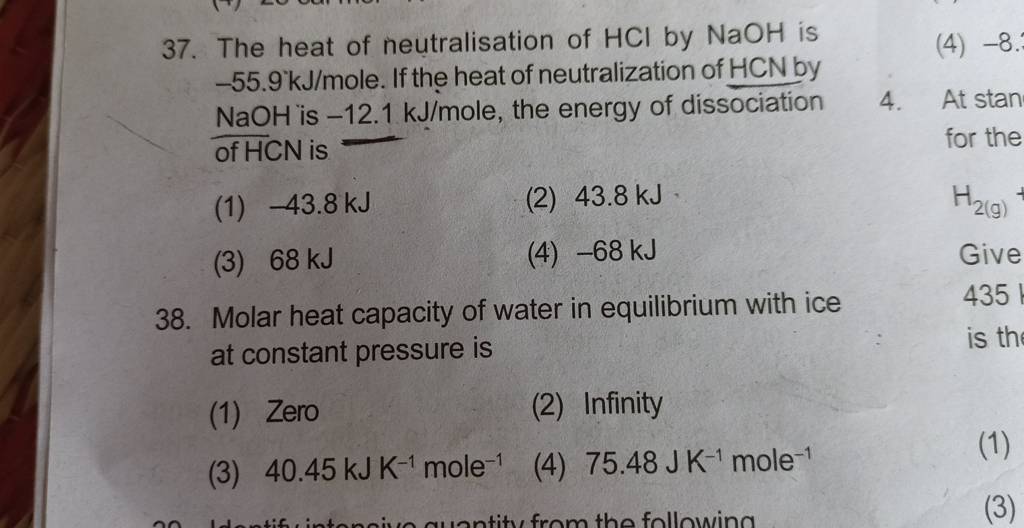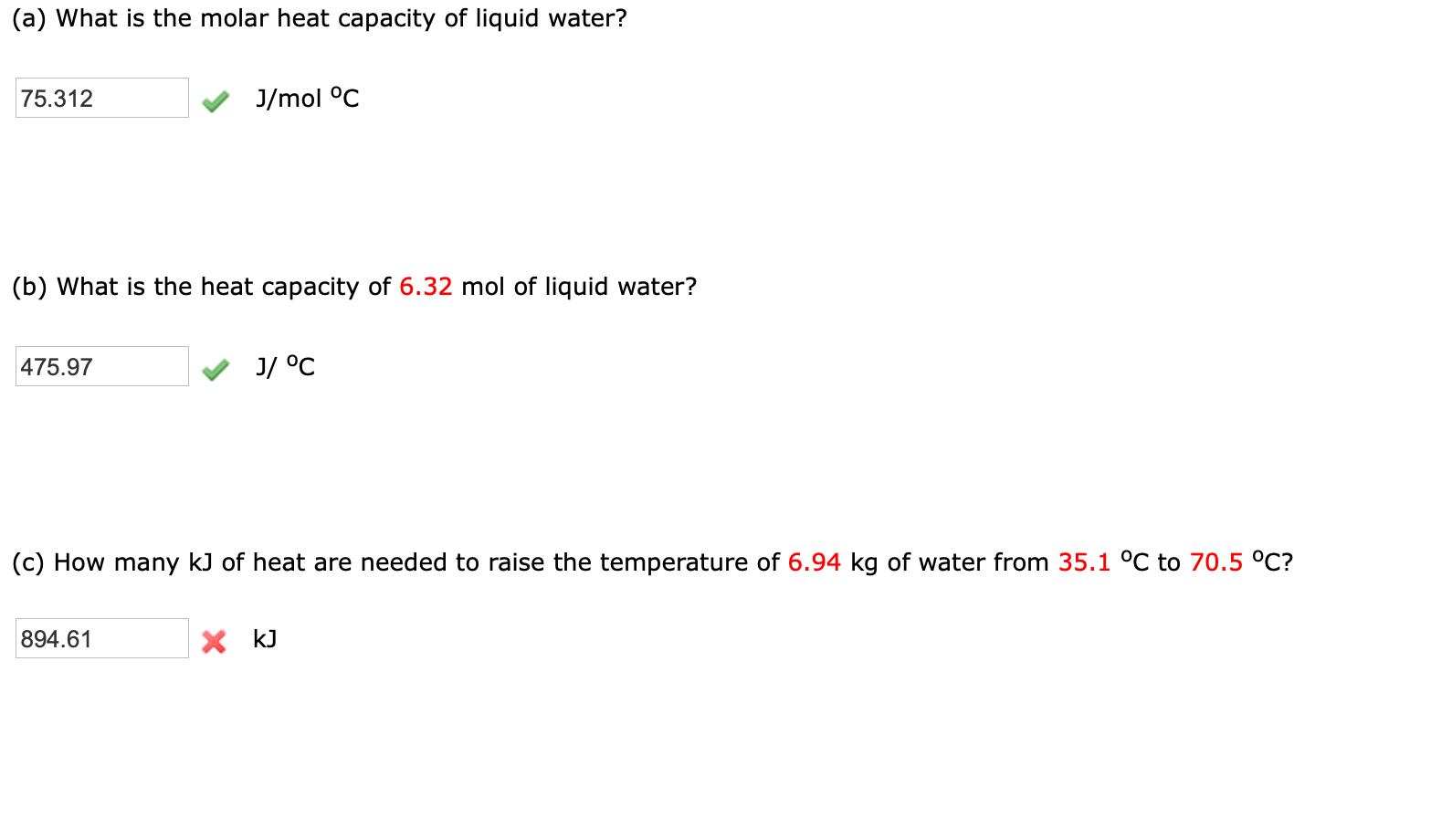What Is The Molar Heat Capacity Of Liquid Water
What Is The Molar Heat Capacity Of Liquid Water - The molar heat capacity of liquid water is 75.348 j/mol k. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g c. The molar heat capacity of liquid water is approximately 75.3 j/mol· c. It is calculated as the product of the specific heat capacity of liquid. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. This means that it takes 75.3 joules of energy to raise the. What is the smallest number.
The molar heat capacity of liquid water is 75.348 j/mol k. This means that it takes 75.3 joules of energy to raise the. It is calculated as the product of the specific heat capacity of liquid. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. What is the smallest number. The molar heat capacity of liquid water is approximately 75.3 j/mol· c. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g c.
This means that it takes 75.3 joules of energy to raise the. The molar heat capacity of liquid water is 75.348 j/mol k. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g c. It is calculated as the product of the specific heat capacity of liquid. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. What is the smallest number. The molar heat capacity of liquid water is approximately 75.3 j/mol· c.
SOLVED The molar heat capacity (Cp) of liquid water is 75.3 J/molK. If
The molar heat capacity of liquid water is approximately 75.3 j/mol· c. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. The molar heat capacity of liquid water is 75.348 j/mol k. It is calculated as the product of the specific heat capacity of liquid. This means.
Molar heat capacity of water in equilibrium with ice at constant pressure..
Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. The molar heat capacity of liquid water is approximately 75.3 j/mol· c. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g c. It is calculated as the product of.
Solved (a) What is the molar heat capacity of liquid water?
The molar heat capacity of liquid water is 75.348 j/mol k. It is calculated as the product of the specific heat capacity of liquid. What is the smallest number. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g c. Online calculator, figures and tables showing specific heat, c p and c.
Q) molar heat capacity of pure water is 18cal per mole Keluen. A vessel f..
The molar heat capacity of liquid water is 75.348 j/mol k. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. It is calculated as the product of the specific heat capacity of liquid. What is the smallest number. This means that it takes 75.3 joules of energy.
Water Heat Capacity Equation Tessshebaylo
What is the smallest number. The molar heat capacity of liquid water is 75.348 j/mol k. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. This means that it takes 75.3 joules of energy to raise the. For liquid at room temperature and pressure, the value of.
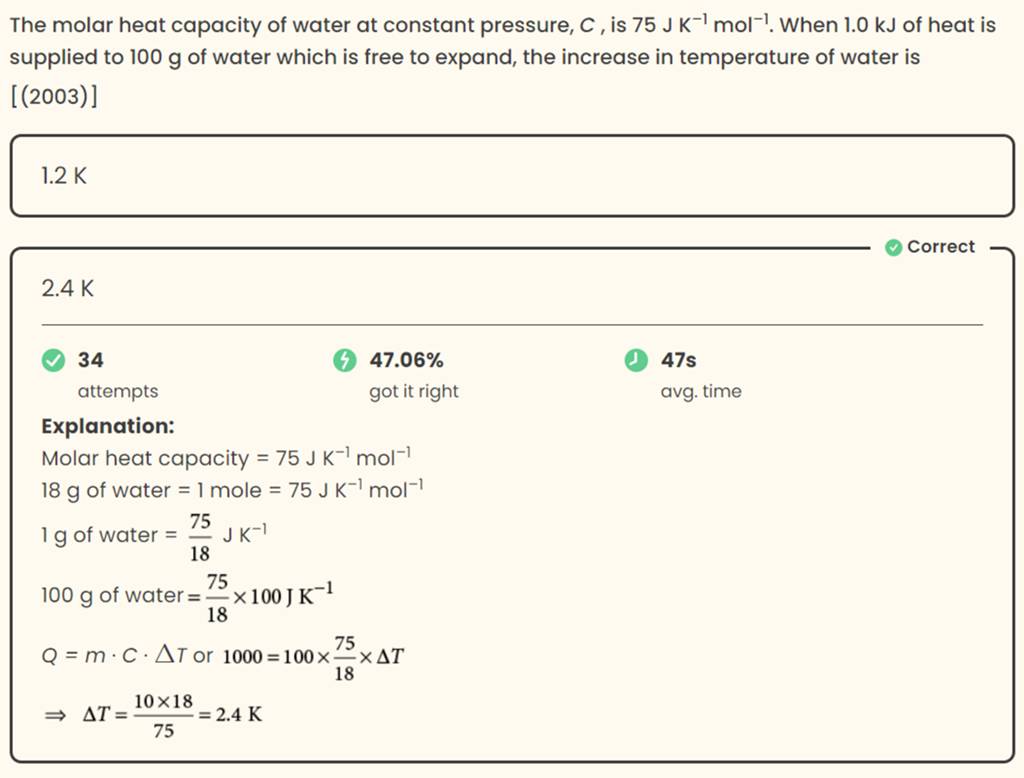
The molar heat capacity of water at constant pressure, C, is 75 J K−1 mol..
It is calculated as the product of the specific heat capacity of liquid. The molar heat capacity of liquid water is 75.348 j/mol k. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g c. The molar heat capacity of liquid water is approximately 75.3 j/mol· c. This means that it takes.
Figure 7 from Molar Heat Capacity (Cv) for Saturated and Compressed
The molar heat capacity of liquid water is approximately 75.3 j/mol· c. It is calculated as the product of the specific heat capacity of liquid. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. For liquid at room temperature and pressure, the value of specific heat capacity.
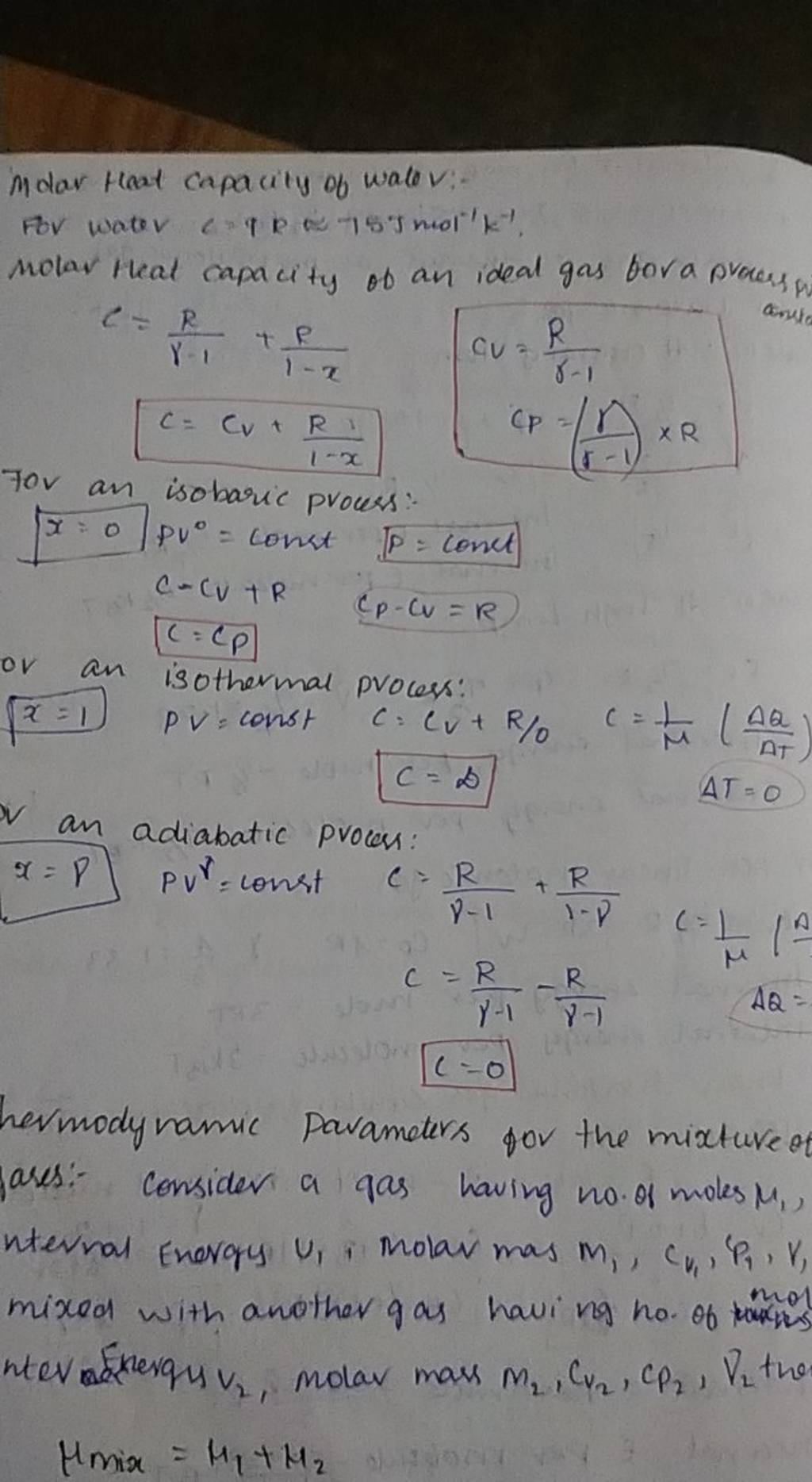
Molar Heat capacity of wale V For water c=9R≈75 m mol −1k−1. molar that..
The molar heat capacity of liquid water is approximately 75.3 j/mol· c. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. What is the smallest number. The molar heat capacity of liquid water is 75.348 j/mol k. It is calculated as the product of the specific heat.
SOLVED The specific heat of water is 4.18 J/(g⋅°C). Calculate the
The molar heat capacity of liquid water is 75.348 j/mol k. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g c. It is calculated as the product of the specific heat capacity of liquid. The molar heat capacity of liquid water is approximately 75.3 j/mol· c. What is the smallest number.
What Is The Molar Heat Capacity Of Water Unraveling Its Thermal Mysteries
The molar heat capacity of liquid water is approximately 75.3 j/mol· c. This means that it takes 75.3 joules of energy to raise the. What is the smallest number. The molar heat capacity of liquid water is 75.348 j/mol k. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures.
It Is Calculated As The Product Of The Specific Heat Capacity Of Liquid.
The molar heat capacity of liquid water is approximately 75.3 j/mol· c. Online calculator, figures and tables showing specific heat, c p and c v, of gasous and liquid ammonia at temperatures ranging from. This means that it takes 75.3 joules of energy to raise the. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g c.
The Molar Heat Capacity Of Liquid Water Is 75.348 J/Mol K.
What is the smallest number.