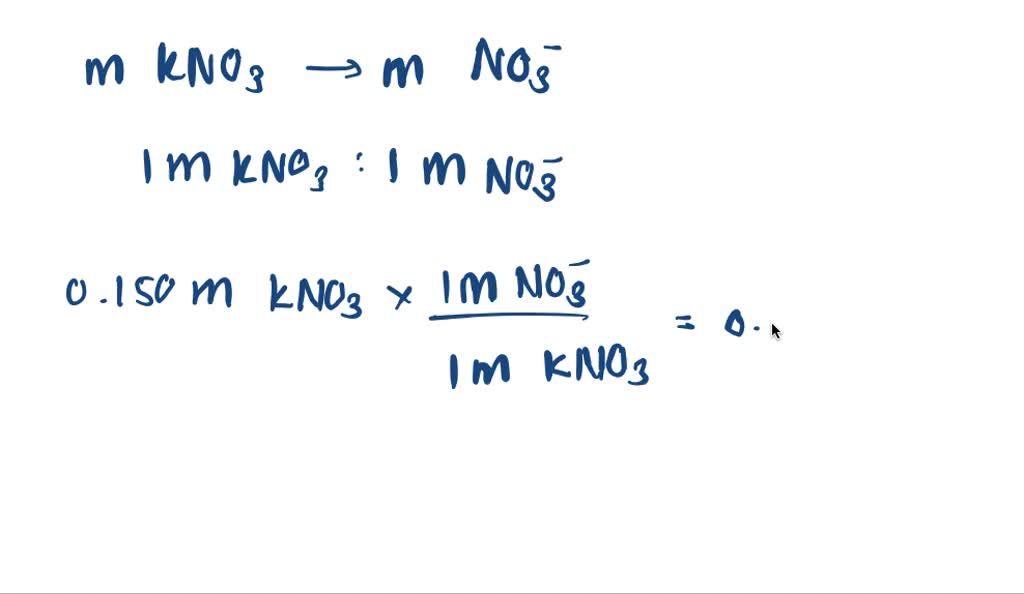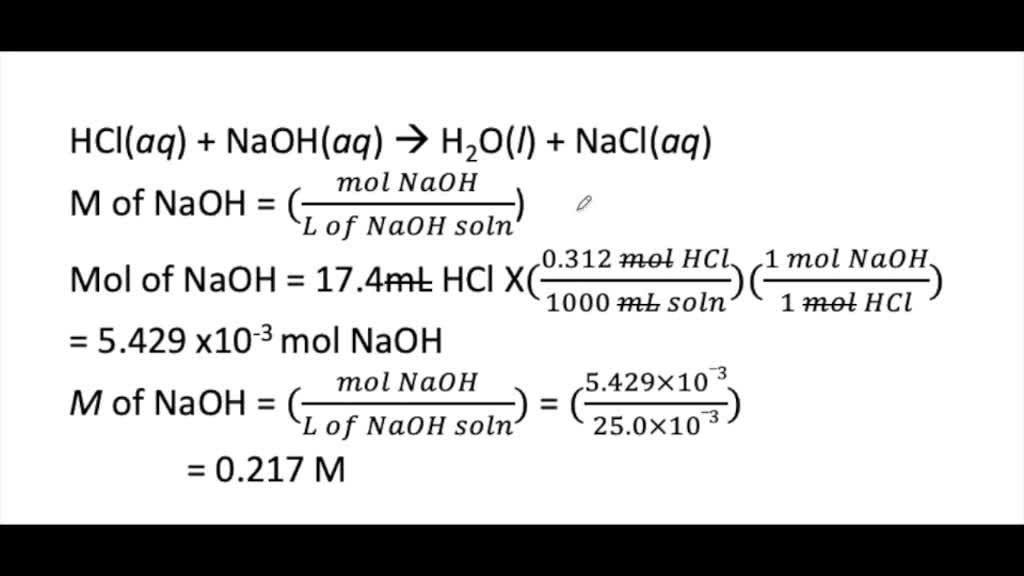What Is The Molarity Of No3 In Each Solution
What Is The Molarity Of No3 In Each Solution - As we learned previously, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the. Therefore, the molarity of no3− in each solution can be calculated as follows: (a) given, molarity of kno a 3, = 0.150 m. Be the first to ask a question about this topic. That's no three negative in each solution. Which of the following solutions will. For part one, we have 0.250 molar of sodium nitrate. For part two, we have 0.250 molar of barium nitrate. Kno a 3 k a + + no a 3 a − What is the molarity of no3− in each solution?
For part two, we have 0.250 molar of barium nitrate. Al (no3)3 has 3 no3− ions. What is the molarity of no3− in each solution? Kno a 3 k a + + no a 3 a − For part one, we have 0.250 molar of sodium nitrate. In 0.300 m al(no3)3, there are three no3. That's no three negative in each solution. Molarity of no3− in 0.120m kno3. As we learned previously, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the. The dissociation of kno a 3 goes like.
Molarity of no3− in 0.120m kno3. Which of the following solutions will. In 0.300 m al(no3)3, there are three no3. For part one, we have 0.250 molar of sodium nitrate. Kno a 3 k a + + no a 3 a − Therefore, the molarity of no3− in each solution can be calculated as follows: As we learned previously, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the. (a) given, molarity of kno a 3, = 0.150 m. What is the molarity of no3− in each solution? That's no three negative in each solution.
SOLVED Molarities of Solutions Solution Molarity NaOH 1.0 M Molarity HCl 0.1 M Molarity HClO
Al (no3)3 has 3 no3− ions. In 0.300 m al(no3)3, there are three no3. Kno a 3 k a + + no a 3 a − As we learned previously, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the. For part one, we have 0.250 molar of sodium nitrate.
What is the molarity of NO3 in each solution? a. 0.150 M KNO3 b. 0.150 M Ca(NO3)2 c. 0.150 M Al
Kno a 3 k a + + no a 3 a − In 0.300 m al(no3)3, there are three no3. For part two, we have 0.250 molar of barium nitrate. Be the first to ask a question about this topic. (a) given, molarity of kno a 3, = 0.150 m.
SOLVED What is the molarity of NO3 in each solution? 0.140 M KNO3. 0.300 M Ca(NO3)2. 0.390 M
Be the first to ask a question about this topic. Kno a 3 k a + + no a 3 a − Which of the following solutions will. Molarity of no3− in 0.120m kno3. For part two, we have 0.250 molar of barium nitrate.
SOLVEDCalculate the concentration (in molarity) of an mathrm{NaOH} solution if 25.0 mathrm
Al (no3)3 has 3 no3− ions. Therefore, the molarity of no3− in each solution can be calculated as follows: (a) given, molarity of kno a 3, = 0.150 m. For part two, we have 0.250 molar of barium nitrate. As we learned previously, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the.
SOLVEDIf 96.3 mL of lead(II) nitrate solution reacts completely with excess sodium iodide
(a) given, molarity of kno a 3, = 0.150 m. The dissociation of kno a 3 goes like. Molarity of no3− in 0.120m kno3. That's no three negative in each solution. For part two, we have 0.250 molar of barium nitrate.
SOLVEDWhat is the molarity of NO3^ in each solution? a. 0.150 MNO3 b. 0.150 MCa(NO3)2 c. 0.150
(a) given, molarity of kno a 3, = 0.150 m. The dissociation of kno a 3 goes like. That's no three negative in each solution. Al (no3)3 has 3 no3− ions. Which of the following solutions will.
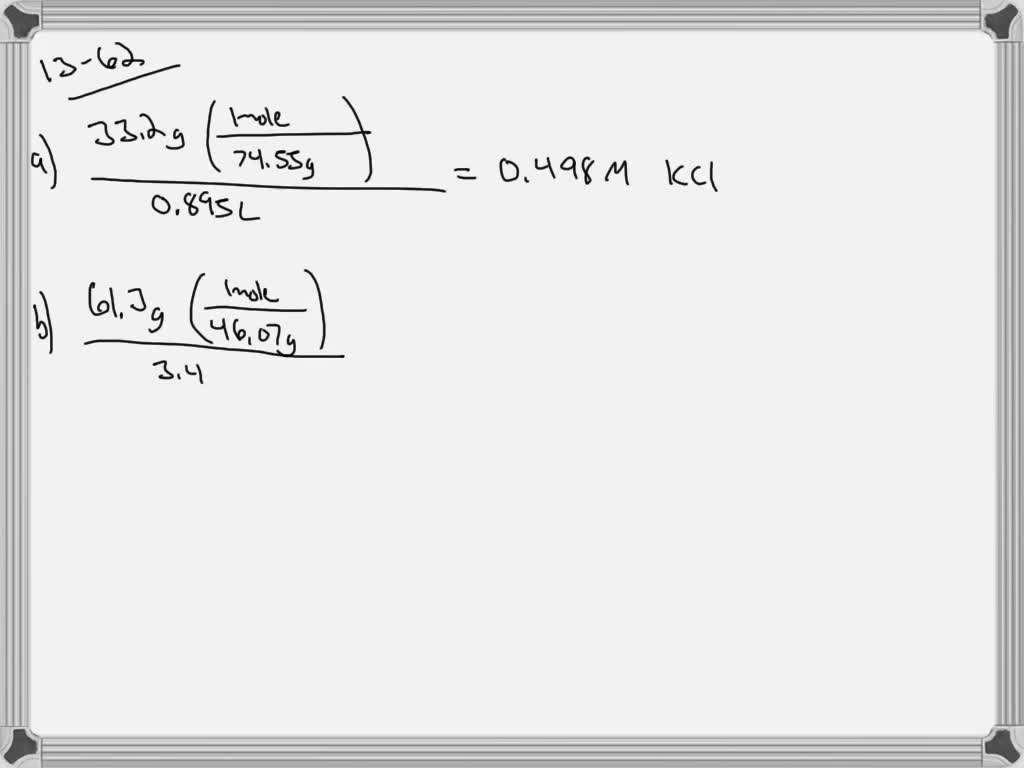
Calculate the molarity of each solution.Part A 33.2 g… SolvedLib
What is the molarity of no3− in each solution? Therefore, the molarity of no3− in each solution can be calculated as follows: Kno a 3 k a + + no a 3 a − (a) given, molarity of kno a 3, = 0.150 m. Molarity of no3− in 0.120m kno3.
[Solved] Calculate the molarity of the Pb(NO3)2(aq) solution.. A solution... Course Hero
Which of the following solutions will. Kno a 3 k a + + no a 3 a − The dissociation of kno a 3 goes like. Therefore, the molarity of no3− in each solution can be calculated as follows: As we learned previously, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the.
SOLVEDWhat is the molarity of NO3^ in each solution?
That's no three negative in each solution. In 0.300 m al(no3)3, there are three no3. Therefore, the molarity of no3− in each solution can be calculated as follows: Be the first to ask a question about this topic. Al (no3)3 has 3 no3− ions.
Molarity Of No3− In 0.120M Kno3.
Which of the following solutions will. Therefore, the molarity of no3− in each solution can be calculated as follows: What is the molarity of no3− in each solution? Al (no3)3 has 3 no3− ions.
That's No Three Negative In Each Solution.
As we learned previously, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the. Be the first to ask a question about this topic. In 0.300 m al(no3)3, there are three no3. Kno a 3 k a + + no a 3 a −
The Dissociation Of Kno A 3 Goes Like.
(a) given, molarity of kno a 3, = 0.150 m. For part two, we have 0.250 molar of barium nitrate. For part one, we have 0.250 molar of sodium nitrate.