What Is The Molecular Geometry Of Ph3
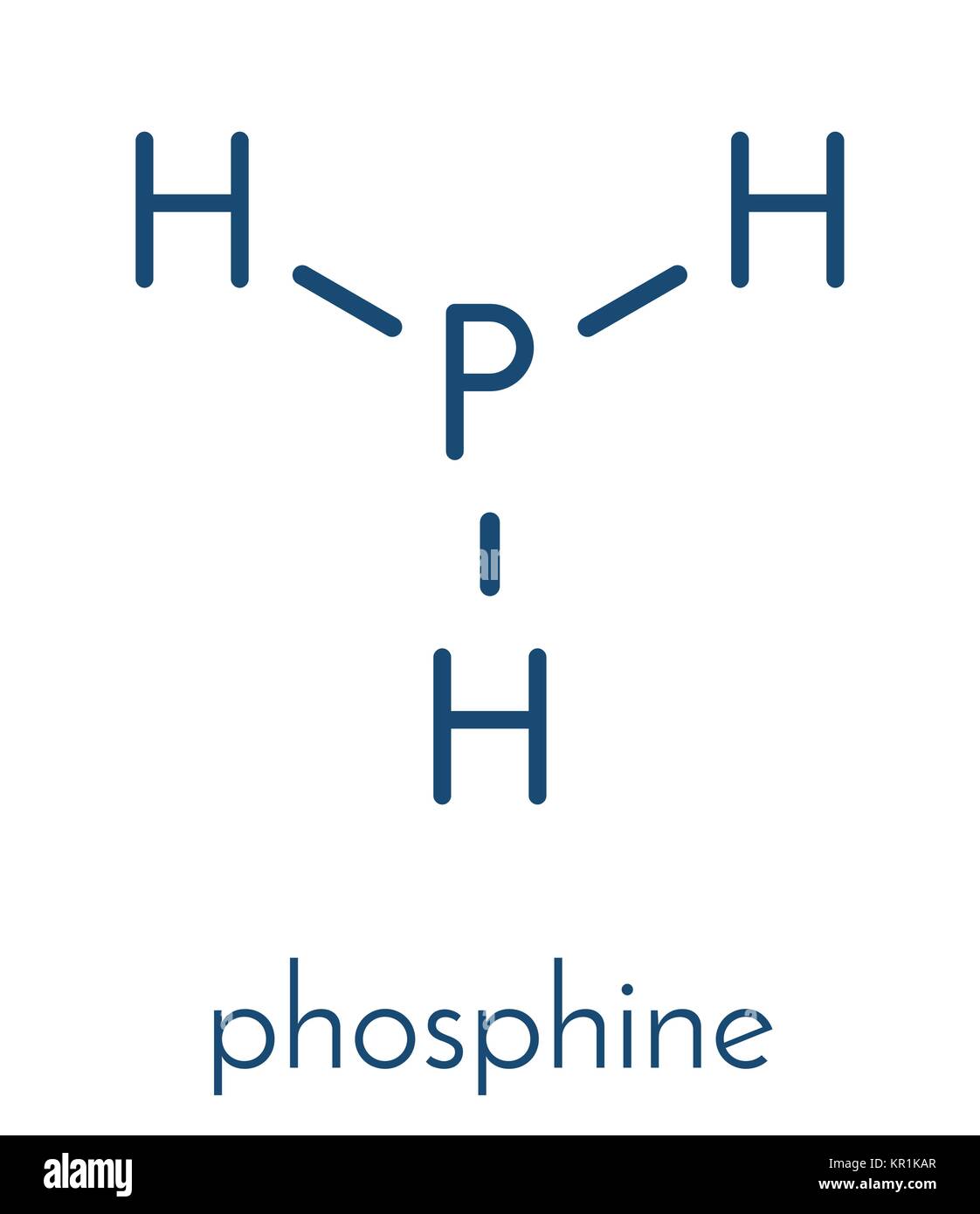
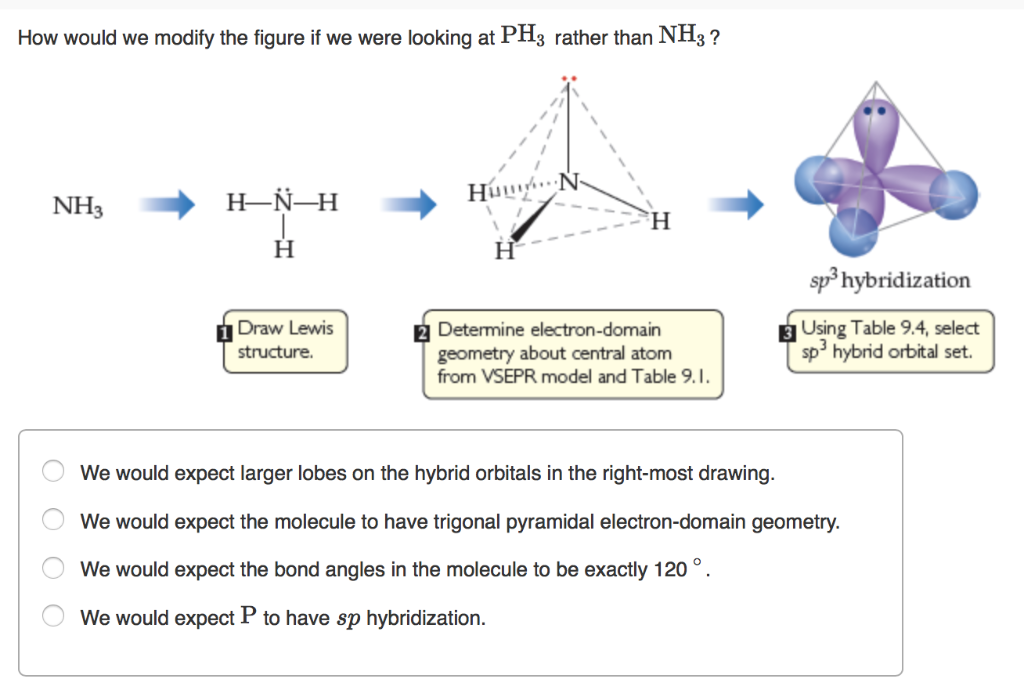
What Is The Molecular Geometry Of Ph3 - The molecular geometry of ph3 (phosphine) is trigonal pyramidal. The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone. Phosphine (ph3) has a central phosphorus atom bonded to.
Phosphine (ph3) has a central phosphorus atom bonded to. The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone. The molecular geometry of ph3 (phosphine) is trigonal pyramidal.
Phosphine (ph3) has a central phosphorus atom bonded to. The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone. The molecular geometry of ph3 (phosphine) is trigonal pyramidal.
Molecular Geometry Of Ph3 Asking List
The molecular geometry of ph3 (phosphine) is trigonal pyramidal. The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone. Phosphine (ph3) has a central phosphorus atom bonded to.
Ph3 molecular geometry subtitlerocket
The molecular geometry of ph3 (phosphine) is trigonal pyramidal. Phosphine (ph3) has a central phosphorus atom bonded to. The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone.
Molecular Geometry Of Ph3 Asking List
The molecular geometry of ph3 (phosphine) is trigonal pyramidal. Phosphine (ph3) has a central phosphorus atom bonded to. The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone.
Ph3 molecular geometry mytejade
The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone. Phosphine (ph3) has a central phosphorus atom bonded to. The molecular geometry of ph3 (phosphine) is trigonal pyramidal.
Ph3 molecular geometry vacationglop
Phosphine (ph3) has a central phosphorus atom bonded to. The molecular geometry of ph3 (phosphine) is trigonal pyramidal. The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone.
Ph3 molecular geometry subtitlerocket
The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone. The molecular geometry of ph3 (phosphine) is trigonal pyramidal. Phosphine (ph3) has a central phosphorus atom bonded to.
Ph3 molecular geometry inrikobold
The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone. Phosphine (ph3) has a central phosphorus atom bonded to. The molecular geometry of ph3 (phosphine) is trigonal pyramidal.
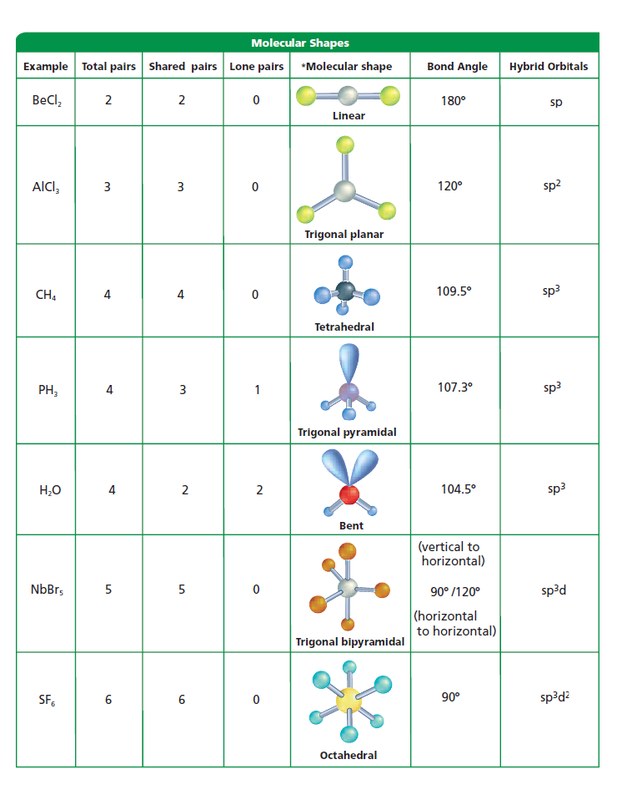
Molecular Geometry Definition, Chart, Shapes, and Examples
The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone. The molecular geometry of ph3 (phosphine) is trigonal pyramidal. Phosphine (ph3) has a central phosphorus atom bonded to.
Molecular Geometry Of Ph3 Asking List
The molecular geometry of ph3 (phosphine) is trigonal pyramidal. The molecular geometry of ph₃ is trigonal pyramidal, featuring three hydrogen atoms bonded to a central phosphorus atom with one lone. Phosphine (ph3) has a central phosphorus atom bonded to.
The Molecular Geometry Of Ph₃ Is Trigonal Pyramidal, Featuring Three Hydrogen Atoms Bonded To A Central Phosphorus Atom With One Lone.
Phosphine (ph3) has a central phosphorus atom bonded to. The molecular geometry of ph3 (phosphine) is trigonal pyramidal.