What Is The Molecular Geometry Of Sih4
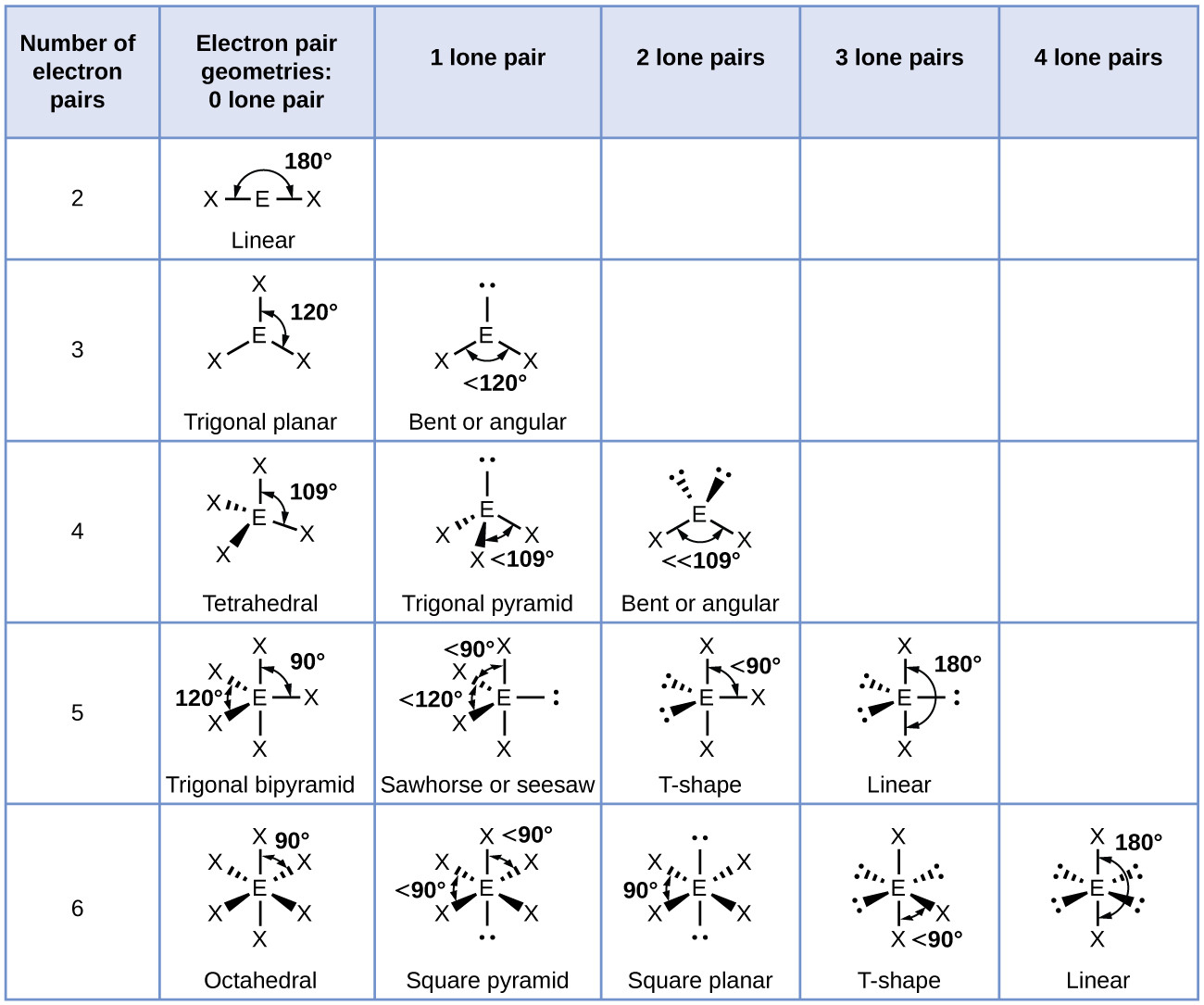
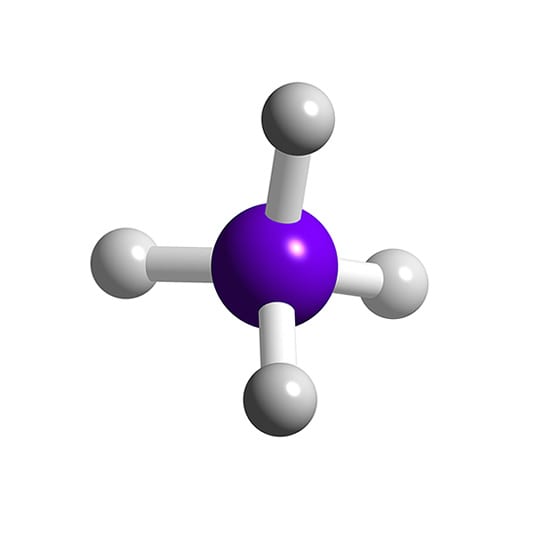
What Is The Molecular Geometry Of Sih4 - The molecular geometry of sih4 (silane) is tetrahedral. This is because sih4 has a central silicon atom surrounded by four. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). Its lewis structure depicts the arrangement of.
Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). The molecular geometry of sih4 (silane) is tetrahedral. This is because sih4 has a central silicon atom surrounded by four. Its lewis structure depicts the arrangement of.
The molecular geometry of sih4 (silane) is tetrahedral. Its lewis structure depicts the arrangement of. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). This is because sih4 has a central silicon atom surrounded by four.
[Solved] draw lewis structure and molecular geometry of xeo4 SO2CL2
The molecular geometry of sih4 (silane) is tetrahedral. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). Its lewis structure depicts the arrangement of. This is because sih4 has a central silicon atom surrounded by four.
Sih4 Lewis Structure Molecular Geometry
Its lewis structure depicts the arrangement of. This is because sih4 has a central silicon atom surrounded by four. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). The molecular geometry of sih4 (silane) is tetrahedral.
Molecular Geometry and Lewis Structure of Sulfur Tetrafluoride (SF4)
This is because sih4 has a central silicon atom surrounded by four. The molecular geometry of sih4 (silane) is tetrahedral. Its lewis structure depicts the arrangement of. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h).
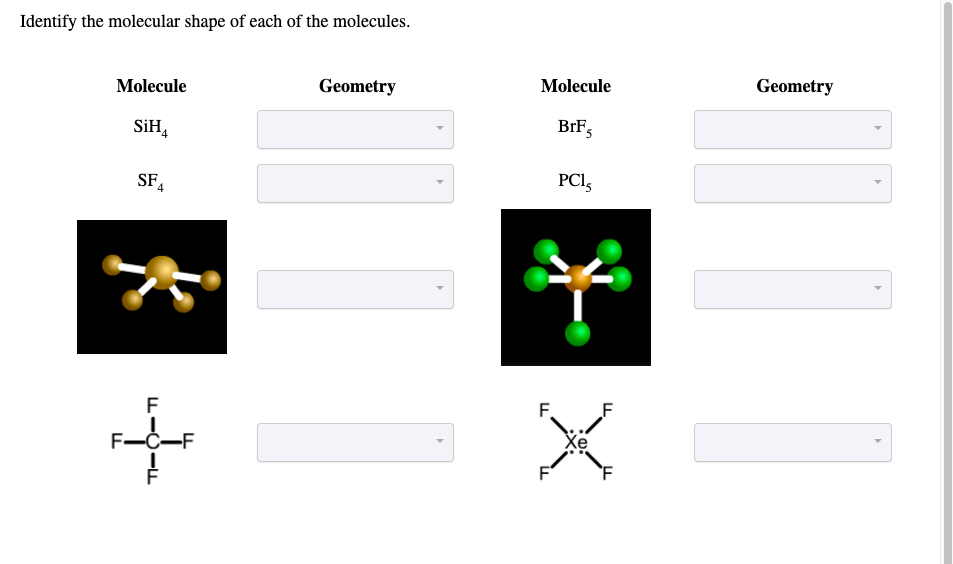
Solved Identify the molecular shape of each of the
Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). The molecular geometry of sih4 (silane) is tetrahedral. This is because sih4 has a central silicon atom surrounded by four. Its lewis structure depicts the arrangement of.
SOLVED Draw a Lewis structure for silane (SiH4) and predict its
The molecular geometry of sih4 (silane) is tetrahedral. Its lewis structure depicts the arrangement of. This is because sih4 has a central silicon atom surrounded by four. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h).
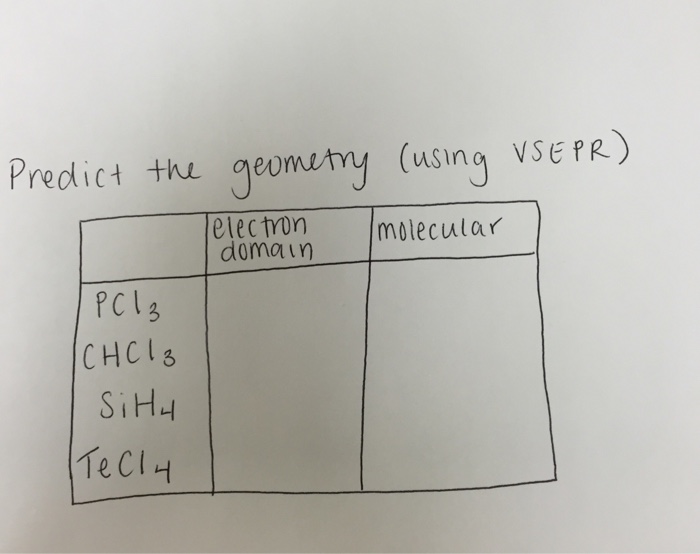
Solved Predict The Geometry (using VSEPR) Electron Domain...
The molecular geometry of sih4 (silane) is tetrahedral. This is because sih4 has a central silicon atom surrounded by four. Its lewis structure depicts the arrangement of. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h).
SOLVED What is the molecular geometry of the SiH4 molecule?
This is because sih4 has a central silicon atom surrounded by four. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). The molecular geometry of sih4 (silane) is tetrahedral. Its lewis structure depicts the arrangement of.
Sih4 Molecular Geometry
Its lewis structure depicts the arrangement of. This is because sih4 has a central silicon atom surrounded by four. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). The molecular geometry of sih4 (silane) is tetrahedral.
Sih4 Molecular Geometry
The molecular geometry of sih4 (silane) is tetrahedral. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). This is because sih4 has a central silicon atom surrounded by four. Its lewis structure depicts the arrangement of.
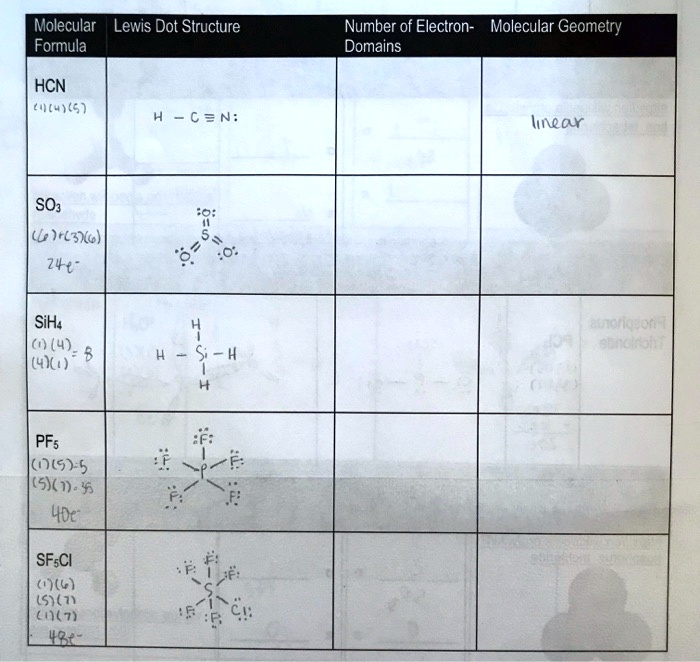
SOLVED Molecular Lewis Dot Structure Formula Number of Electrons
Its lewis structure depicts the arrangement of. The molecular geometry of sih4 (silane) is tetrahedral. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). This is because sih4 has a central silicon atom surrounded by four.
This Is Because Sih4 Has A Central Silicon Atom Surrounded By Four.
The molecular geometry of sih4 (silane) is tetrahedral. Silane (sih4) is a compound composed of silicon (si) and hydrogen (h). Its lewis structure depicts the arrangement of.