What Is The Molecular Shape Of If5
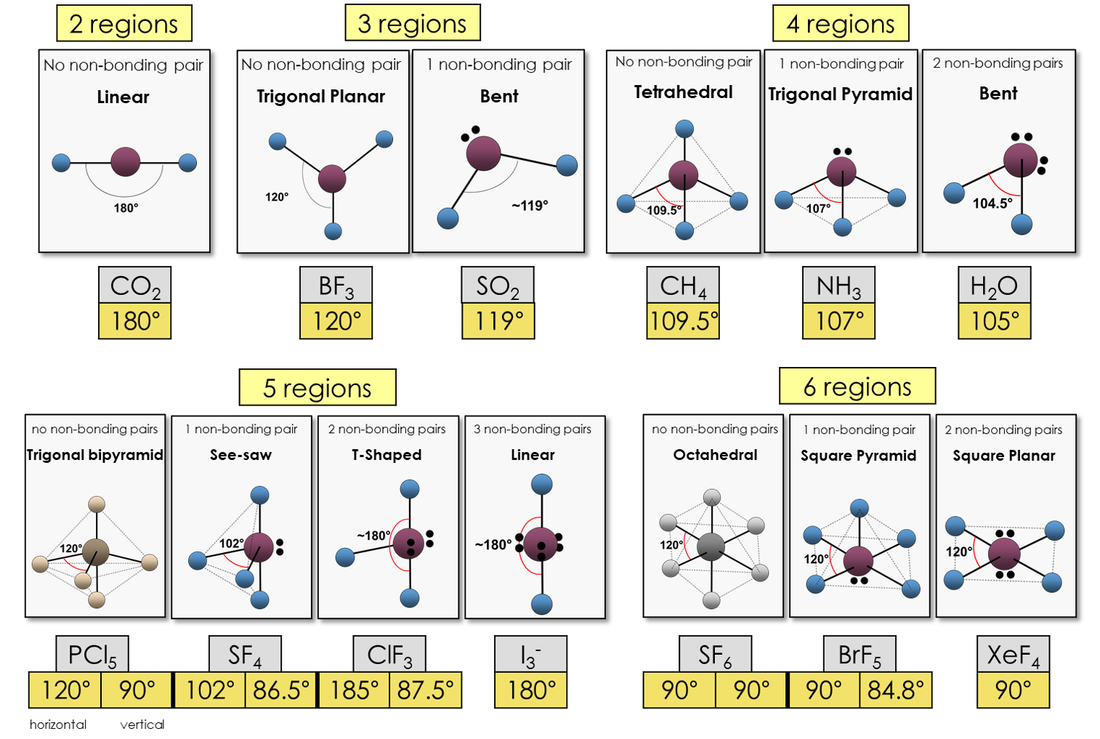
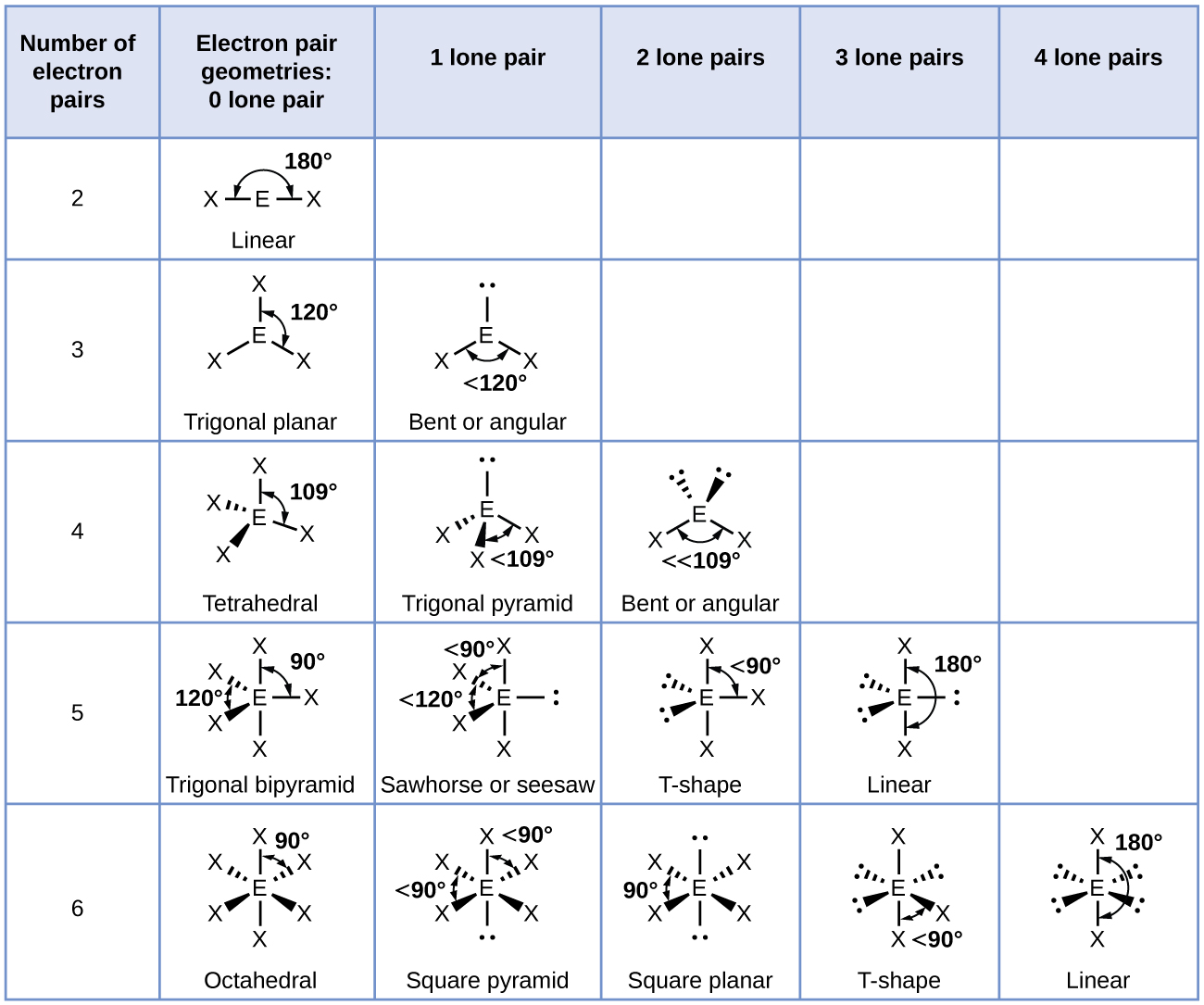
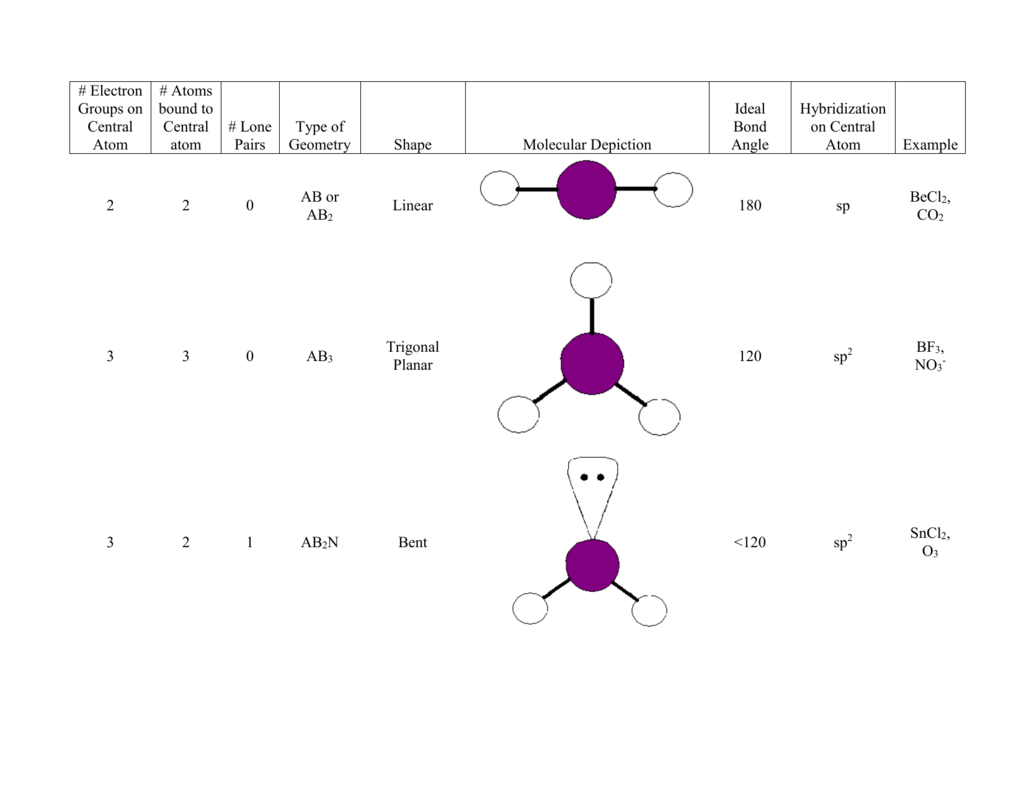
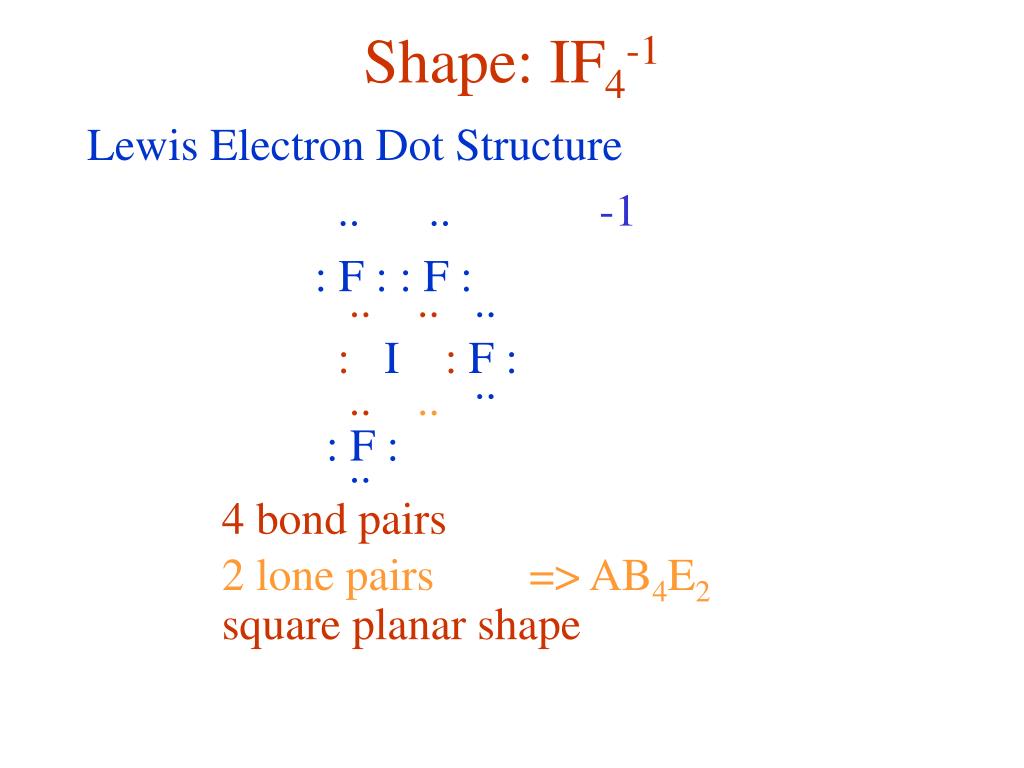
What Is The Molecular Shape Of If5 - The molecular geometry of if5 can be determined using the vsepr theory. A=central atom, x= numbers of atoms bonded to the central atom and=number of lone pairs. The electron geometry for the iodine pentafluoride. The bond angles formed are close to 90°. Vsepr theory explained that if5 is ax 5 e type molecule. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description of the if5 bond angles. The molecular geometry or shape of the if 5 molecule is square pyramidal. The molecular geometry of if5 is square pyramidal. If 5 lewis structure shape. The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons around.
If 5 lewis structure shape. The bond angles formed are close to 90°. A=central atom, x= numbers of atoms bonded to the central atom and=number of lone pairs. The electron geometry for the iodine pentafluoride. The molecular geometry or shape of the if 5 molecule is square pyramidal. The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons around. The molecular geometry of if5 can be determined using the vsepr theory. Vsepr theory explained that if5 is ax 5 e type molecule. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description of the if5 bond angles. The molecular geometry of if5 is square pyramidal.
The molecular geometry or shape of the if 5 molecule is square pyramidal. The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons around. The electron geometry for the iodine pentafluoride. The molecular geometry of if5 can be determined using the vsepr theory. The molecular geometry of if5 is square pyramidal. If 5 lewis structure shape. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description of the if5 bond angles. A=central atom, x= numbers of atoms bonded to the central atom and=number of lone pairs. The bond angles formed are close to 90°. Vsepr theory explained that if5 is ax 5 e type molecule.
SOLVED Text Draw the Lewis structure for the following compounds and
The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons around. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description of the if5 bond angles. The electron geometry for the iodine pentafluoride. Vsepr theory explained that.
If5 Lewis Structure Hybridization Polarity And Molecular Shape guidetech
The bond angles formed are close to 90°. The molecular geometry of if5 can be determined using the vsepr theory. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description of the if5 bond angles. If 5 lewis structure shape. The molecular geometry or shape of the if 5 molecule is square pyramidal.
4. Molecular Shapes
The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons around. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description of the if5 bond angles. The molecular geometry of if5 is square pyramidal. The electron geometry.
5.2 Molecular Shape Chemistry LibreTexts
The molecular geometry of if5 can be determined using the vsepr theory. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description of the if5 bond angles. The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons.
The Geometrical Arrangement Of Electrons And Molecular
Vsepr theory explained that if5 is ax 5 e type molecule. The molecular geometry of if5 can be determined using the vsepr theory. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description of the if5 bond angles. The molecular geometry of if5 is square pyramidal. The electron geometry for the iodine pentafluoride.
If5 Molecular Geometry ma
The molecular geometry of if5 is square pyramidal. The bond angles formed are close to 90°. The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons around. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description.
IF5 Lewis structure, molecular geometry, bond angle, hybridization
The molecular geometry of if5 can be determined using the vsepr theory. The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons around. If 5 lewis structure shape. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a.
PPT Molecular Geometry and Chemical Bonding Theory PowerPoint
The electron geometry for the iodine pentafluoride. The molecular geometry of if5 can be determined using the vsepr theory. An explanation of the molecular geometry for the if5 (iodine pentafluoride) including a description of the if5 bond angles. The bond angles formed are close to 90°. The molecular geometry of if5 is square pyramidal.
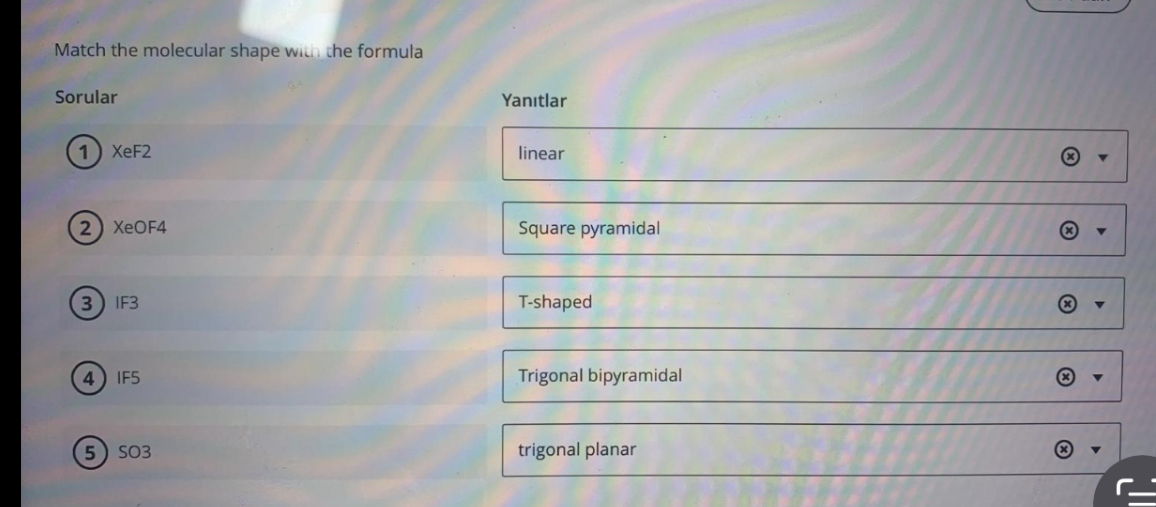
SOLVED Match the molecular shape with the formula Sorular (1) X e F 2
The molecular geometry of if5 is square pyramidal. If 5 lewis structure shape. A=central atom, x= numbers of atoms bonded to the central atom and=number of lone pairs. The electron geometry for the iodine pentafluoride. The molecular geometry of if5 can be determined using the vsepr theory.
Electron domain geometry vs molecular shape chart guglafro
The electron geometry for the iodine pentafluoride. The molecular geometry or shape of the if 5 molecule is square pyramidal. The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons around. The molecular geometry of if5 is square pyramidal. An explanation.
An Explanation Of The Molecular Geometry For The If5 (Iodine Pentafluoride) Including A Description Of The If5 Bond Angles.
The bond angles formed are close to 90°. The molecular geometry of if5 can be determined using the vsepr theory. A=central atom, x= numbers of atoms bonded to the central atom and=number of lone pairs. Vsepr theory explained that if5 is ax 5 e type molecule.
The Molecular Geometry Or Shape Of The If 5 Molecule Is Square Pyramidal.
The electron geometry for the iodine pentafluoride. If 5 lewis structure shape. The molecular geometry of if5 is square pyramidal. The molecular shape of if₅, or iodine pentafluoride, is square pyramidal as determined by vsepr theory due to the arrangements of bonded pairs and a lone pair of electrons around.