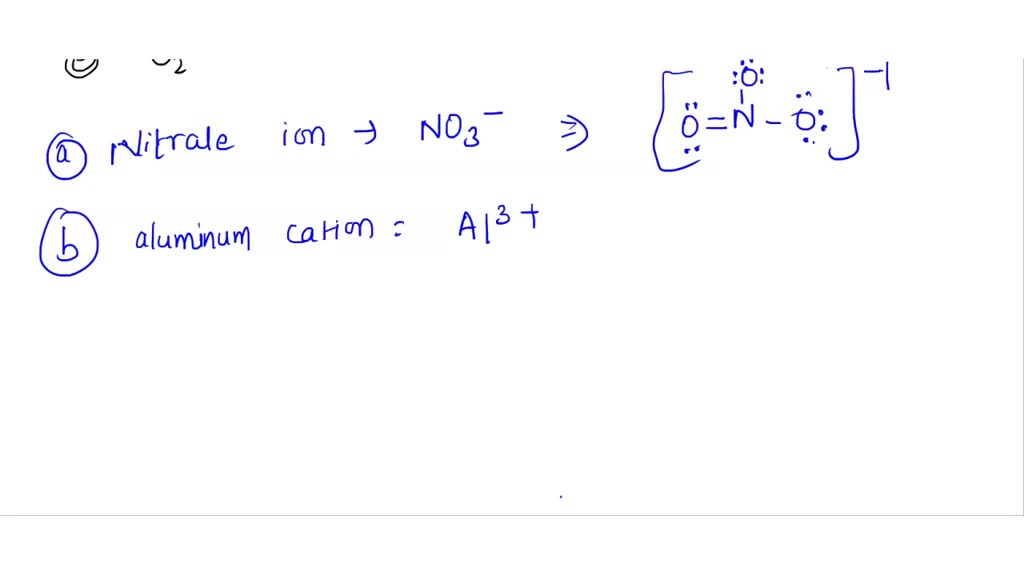What Is The Name Of Al2 So4 3
What Is The Name Of Al2 So4 3 - It is a chemical compound typically used as a coagulating agent for purification of. The correct name for the compound al2(so4)3 is aluminum sulfate and for the compound n2o3 is dinitrogen trioxide. The compound contains two aluminum ions (al³+) and three sulfate ions. The chemical formula of aluminium sulfate is al 2 (so 4) 3. The correct name for the compound al2(so4)3 is aluminum sulfate. The compound al2(so4)3 is called aluminum sulfate. So, it takes 3 of the so 4 anions to balance 2 of the al cations. So, you get al 2 (so.
So, you get al 2 (so. The correct name for the compound al2(so4)3 is aluminum sulfate and for the compound n2o3 is dinitrogen trioxide. It is a chemical compound typically used as a coagulating agent for purification of. The compound al2(so4)3 is called aluminum sulfate. The compound contains two aluminum ions (al³+) and three sulfate ions. The chemical formula of aluminium sulfate is al 2 (so 4) 3. So, it takes 3 of the so 4 anions to balance 2 of the al cations. The correct name for the compound al2(so4)3 is aluminum sulfate.
So, it takes 3 of the so 4 anions to balance 2 of the al cations. The compound al2(so4)3 is called aluminum sulfate. The correct name for the compound al2(so4)3 is aluminum sulfate and for the compound n2o3 is dinitrogen trioxide. The chemical formula of aluminium sulfate is al 2 (so 4) 3. The compound contains two aluminum ions (al³+) and three sulfate ions. So, you get al 2 (so. It is a chemical compound typically used as a coagulating agent for purification of. The correct name for the compound al2(so4)3 is aluminum sulfate.
Which of the following formulas and the name are incorrect? CaS
It is a chemical compound typically used as a coagulating agent for purification of. The compound al2(so4)3 is called aluminum sulfate. So, it takes 3 of the so 4 anions to balance 2 of the al cations. So, you get al 2 (so. The compound contains two aluminum ions (al³+) and three sulfate ions.
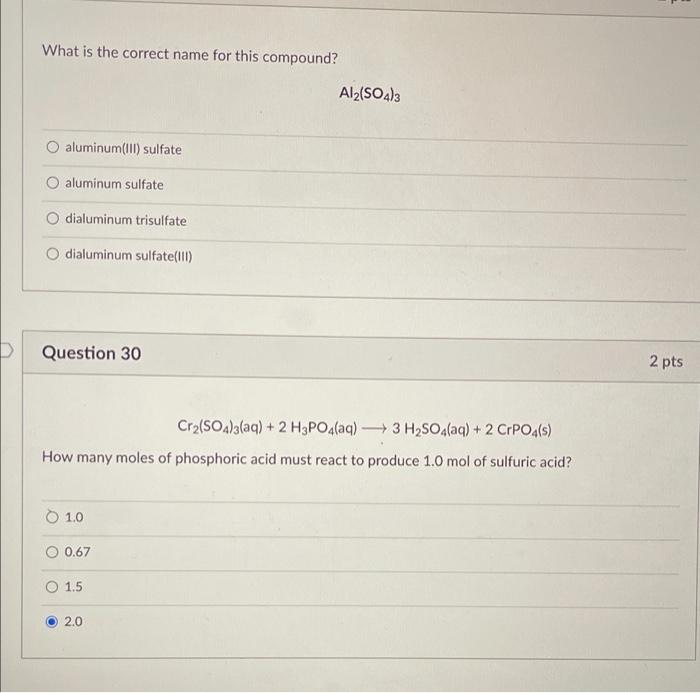
Solved What is the correct name for this compound? Al2(SO4)3
The compound al2(so4)3 is called aluminum sulfate. The correct name for the compound al2(so4)3 is aluminum sulfate. So, you get al 2 (so. So, it takes 3 of the so 4 anions to balance 2 of the al cations. The compound contains two aluminum ions (al³+) and three sulfate ions.
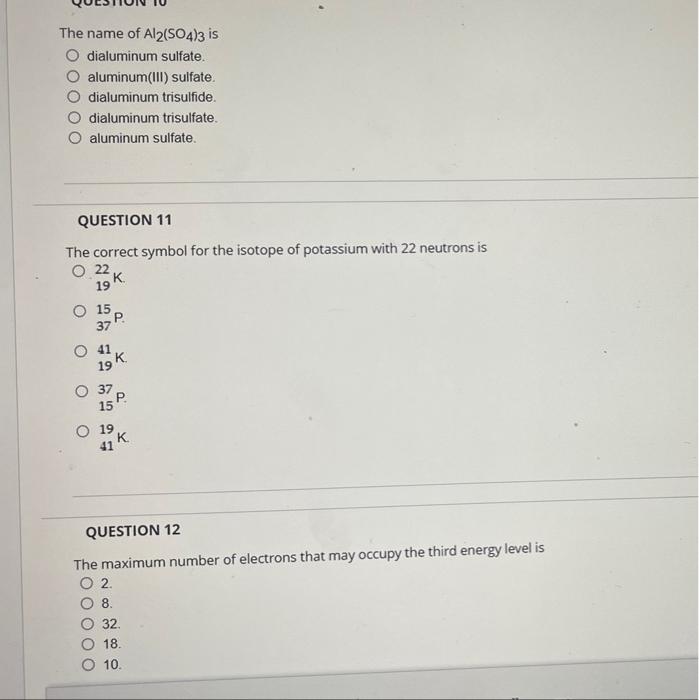
Solved The name of Al2(SO4)3 is dialuminum sulfate.
So, it takes 3 of the so 4 anions to balance 2 of the al cations. The compound contains two aluminum ions (al³+) and three sulfate ions. It is a chemical compound typically used as a coagulating agent for purification of. The chemical formula of aluminium sulfate is al 2 (so 4) 3. The correct name for the compound al2(so4)3.
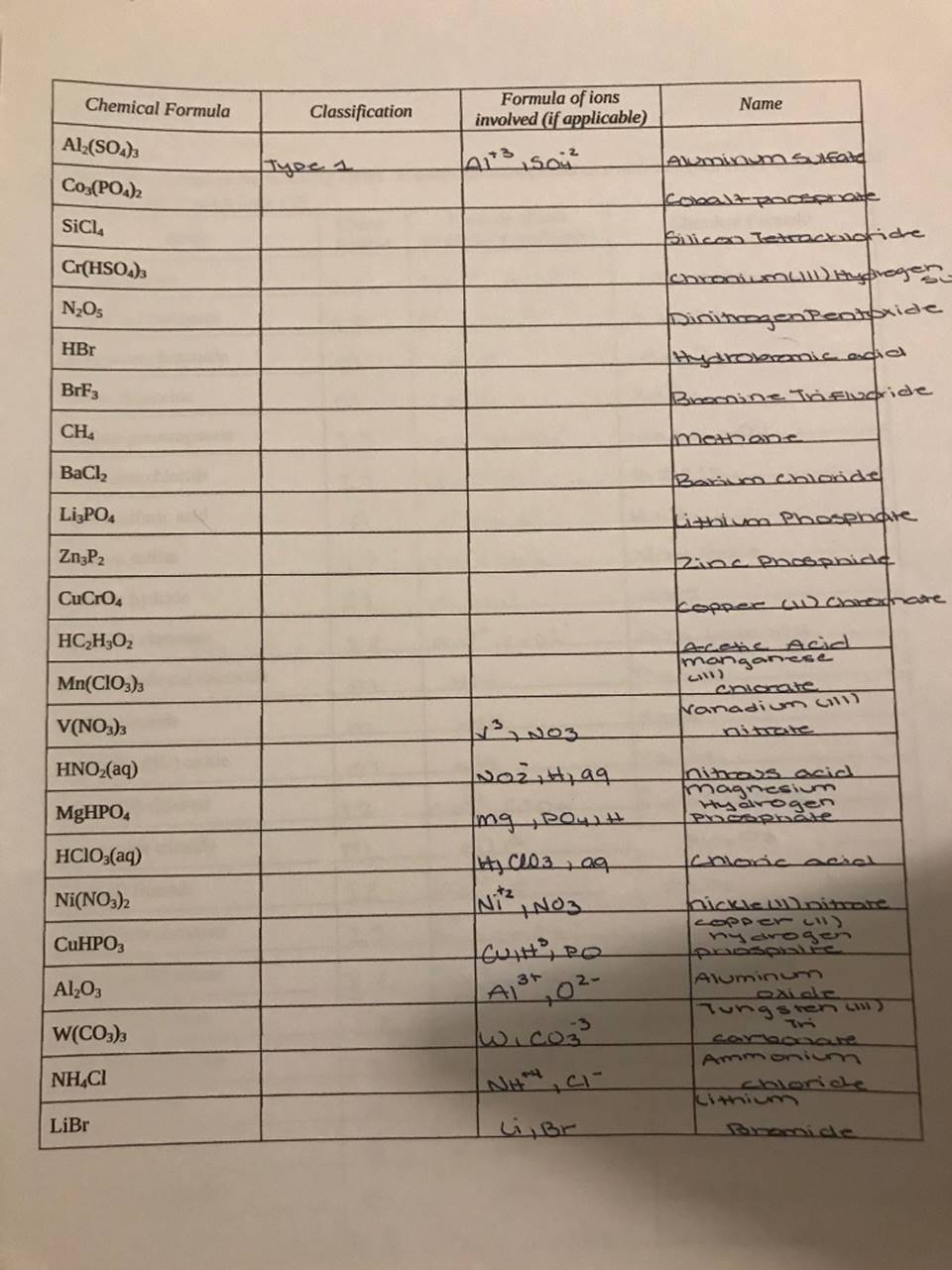
(Solved) Chemical Formula Al2(SO4)3 4113 ???? N203 Are Formula Of
So, you get al 2 (so. The chemical formula of aluminium sulfate is al 2 (so 4) 3. The compound contains two aluminum ions (al³+) and three sulfate ions. The compound al2(so4)3 is called aluminum sulfate. The correct name for the compound al2(so4)3 is aluminum sulfate.
Solved 1) 2 Al(s) + 3 H₂SO4(aq) → Name Al2(SO4)3(aq) + 3
The compound contains two aluminum ions (al³+) and three sulfate ions. The compound al2(so4)3 is called aluminum sulfate. The correct name for the compound al2(so4)3 is aluminum sulfate. So, you get al 2 (so. So, it takes 3 of the so 4 anions to balance 2 of the al cations.
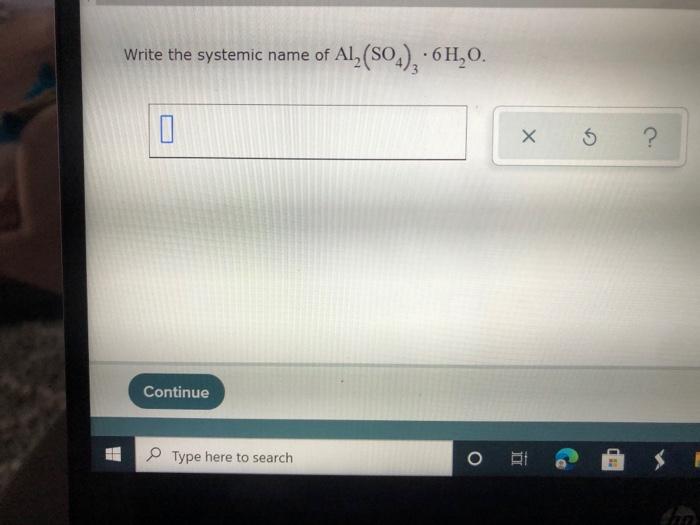
Solved Write the systemic name of Al2(SO4), 6H,0. 0 X 5 ?
The correct name for the compound al2(so4)3 is aluminum sulfate. The compound al2(so4)3 is called aluminum sulfate. The chemical formula of aluminium sulfate is al 2 (so 4) 3. So, it takes 3 of the so 4 anions to balance 2 of the al cations. It is a chemical compound typically used as a coagulating agent for purification of.
(Solved) The Name Of Al2(SO4)3 Is Aluminum Sulfate. 01. Aluminum(III
It is a chemical compound typically used as a coagulating agent for purification of. The correct name for the compound al2(so4)3 is aluminum sulfate. So, it takes 3 of the so 4 anions to balance 2 of the al cations. The compound contains two aluminum ions (al³+) and three sulfate ions. So, you get al 2 (so.
Solved The name of Al 2(SO4)3 İs A) aluminum(III) sulfate
The chemical formula of aluminium sulfate is al 2 (so 4) 3. So, you get al 2 (so. The compound al2(so4)3 is called aluminum sulfate. The correct name for the compound al2(so4)3 is aluminum sulfate. So, it takes 3 of the so 4 anions to balance 2 of the al cations.
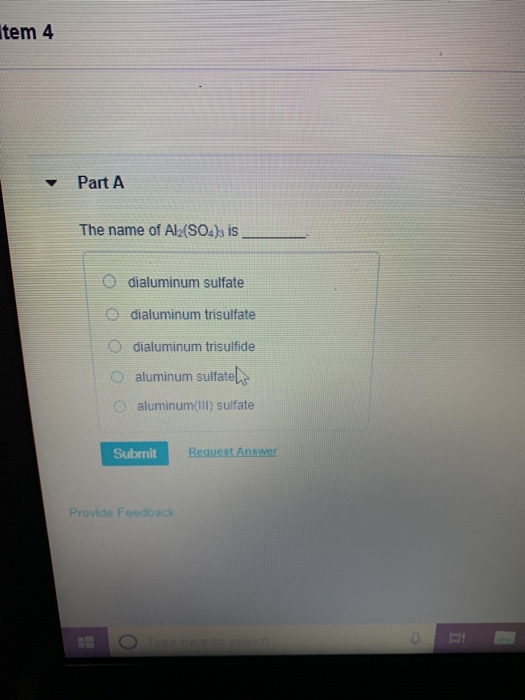
Solved item 4 Part A The name of Al2(SO4)3 is O dialuminum
The compound contains two aluminum ions (al³+) and three sulfate ions. So, you get al 2 (so. The compound al2(so4)3 is called aluminum sulfate. So, it takes 3 of the so 4 anions to balance 2 of the al cations. The correct name for the compound al2(so4)3 is aluminum sulfate and for the compound n2o3 is dinitrogen trioxide.
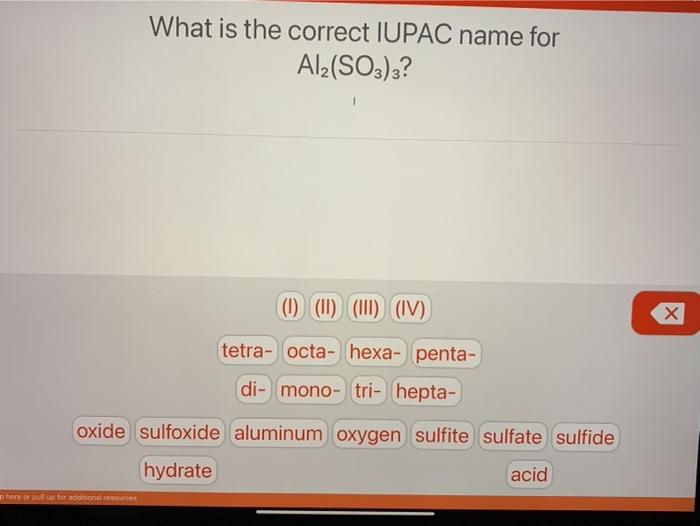
Solved What is the correct IUPAC name for Al2(SO3)3? (1)
So, it takes 3 of the so 4 anions to balance 2 of the al cations. The correct name for the compound al2(so4)3 is aluminum sulfate and for the compound n2o3 is dinitrogen trioxide. The compound al2(so4)3 is called aluminum sulfate. It is a chemical compound typically used as a coagulating agent for purification of. So, you get al 2.
The Compound Al2(So4)3 Is Called Aluminum Sulfate.
So, it takes 3 of the so 4 anions to balance 2 of the al cations. It is a chemical compound typically used as a coagulating agent for purification of. The correct name for the compound al2(so4)3 is aluminum sulfate and for the compound n2o3 is dinitrogen trioxide. So, you get al 2 (so.
The Correct Name For The Compound Al2(So4)3 Is Aluminum Sulfate.
The chemical formula of aluminium sulfate is al 2 (so 4) 3. The compound contains two aluminum ions (al³+) and three sulfate ions.