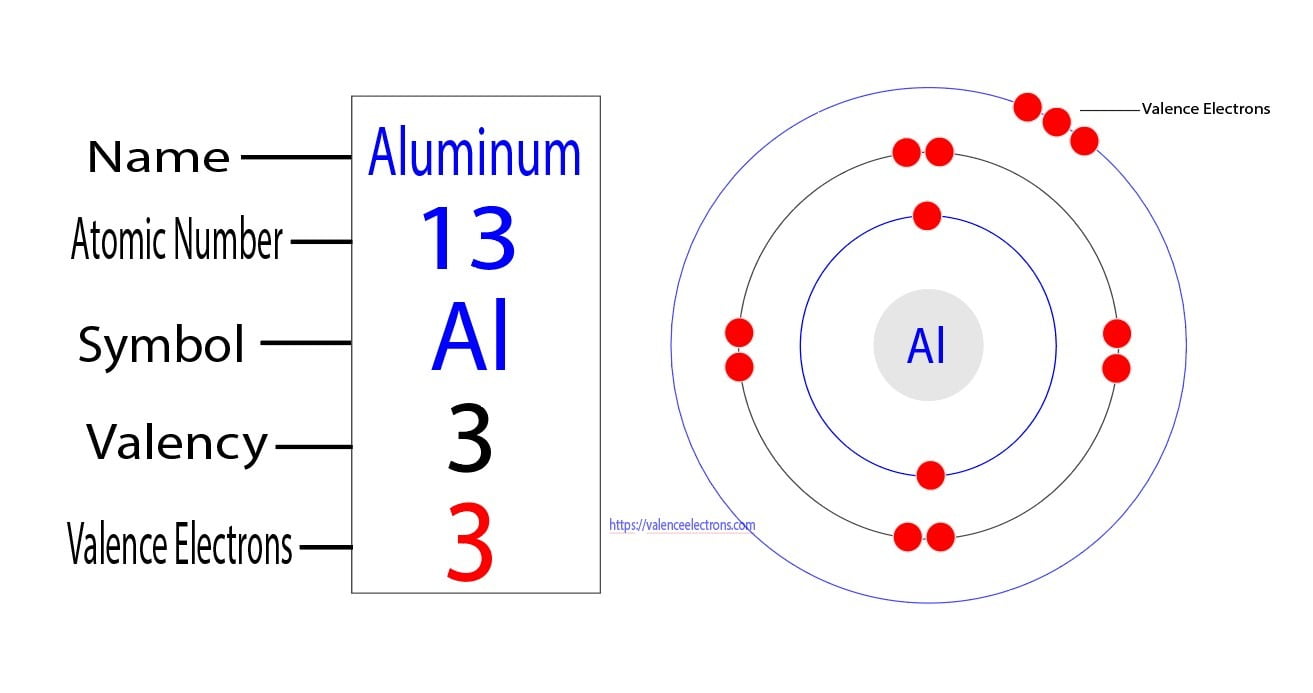
What Is The Number Of Electrons In An Al3 Ion
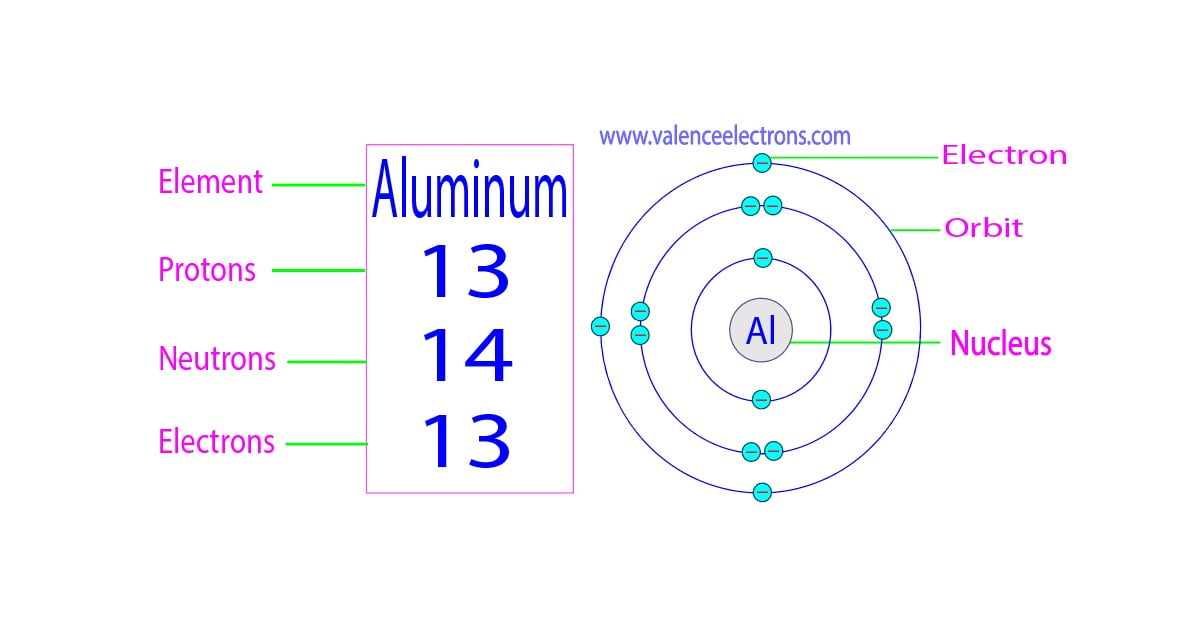
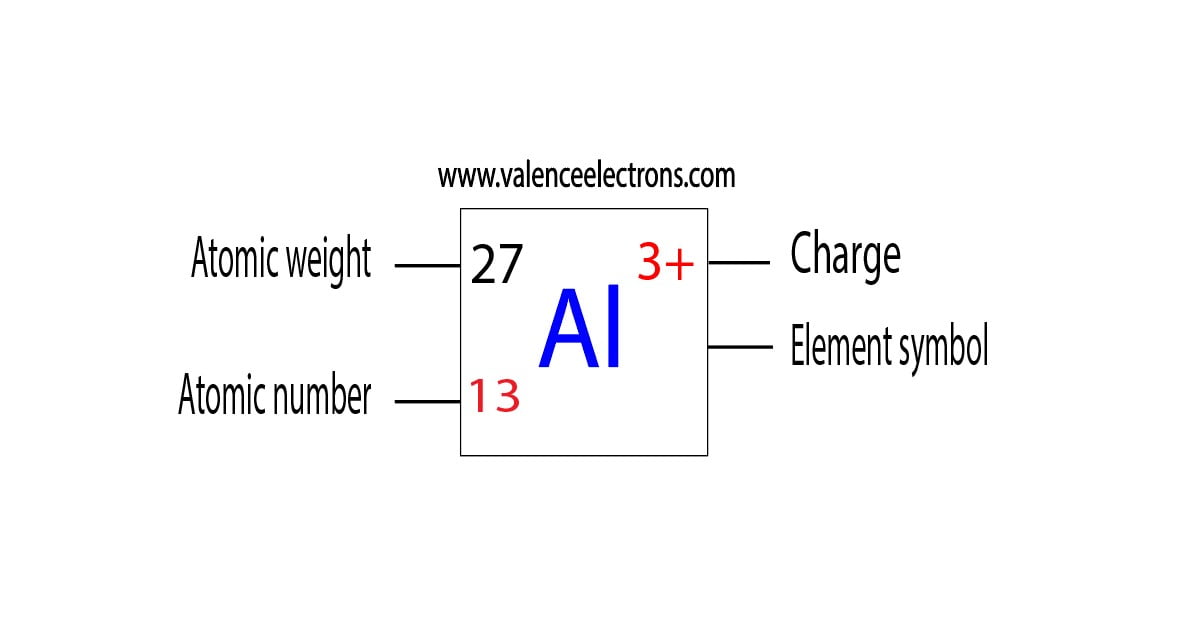
What Is The Number Of Electrons In An Al3 Ion - Having a $ + 3$ charge implies aluminum having an atomic number $13$ has lost three. Clearly, there are 10 electrons associated with a single a l 3 + ion. In a neutral aluminum atom, the number of electrons is equal to the number of protons, so in the al3+ ion, it has 10 electrons. This is an aluminum ion with a charge of $ + 3$. Study with quizlet and memorize flashcards containing terms like what is the number of electrons in an al3+ ion, what occurs when a. An aluminum ion, al3+, has 10 electrons. We don't need any chemistry to make this determination. We don't need any chemistry to make this determination. This is because aluminum normally has 13 protons and 13 electrons in a neutral atom,. Clearly, there are 10 electrons associated with a single al3+ ion.
Clearly, there are 10 electrons associated with a single a l 3 + ion. Clearly, there are 10 electrons associated with a single al3+ ion. We don't need any chemistry to make this determination. We don't need any chemistry to make this determination. An aluminum ion, al3+, has 10 electrons. This is because aluminum normally has 13 protons and 13 electrons in a neutral atom,. In a neutral aluminum atom, the number of electrons is equal to the number of protons, so in the al3+ ion, it has 10 electrons. Study with quizlet and memorize flashcards containing terms like what is the number of electrons in an al3+ ion, what occurs when a. This is an aluminum ion with a charge of $ + 3$. Having a $ + 3$ charge implies aluminum having an atomic number $13$ has lost three.
Clearly, there are 10 electrons associated with a single a l 3 + ion. This is because aluminum normally has 13 protons and 13 electrons in a neutral atom,. Clearly, there are 10 electrons associated with a single al3+ ion. An aluminum ion, al3+, has 10 electrons. In a neutral aluminum atom, the number of electrons is equal to the number of protons, so in the al3+ ion, it has 10 electrons. We don't need any chemistry to make this determination. This is an aluminum ion with a charge of $ + 3$. Having a $ + 3$ charge implies aluminum having an atomic number $13$ has lost three. Study with quizlet and memorize flashcards containing terms like what is the number of electrons in an al3+ ion, what occurs when a. We don't need any chemistry to make this determination.
How many protons, neutrons and electrons does carbon have?
Having a $ + 3$ charge implies aluminum having an atomic number $13$ has lost three. Clearly, there are 10 electrons associated with a single al3+ ion. We don't need any chemistry to make this determination. Clearly, there are 10 electrons associated with a single a l 3 + ion. This is an aluminum ion with a charge of $.
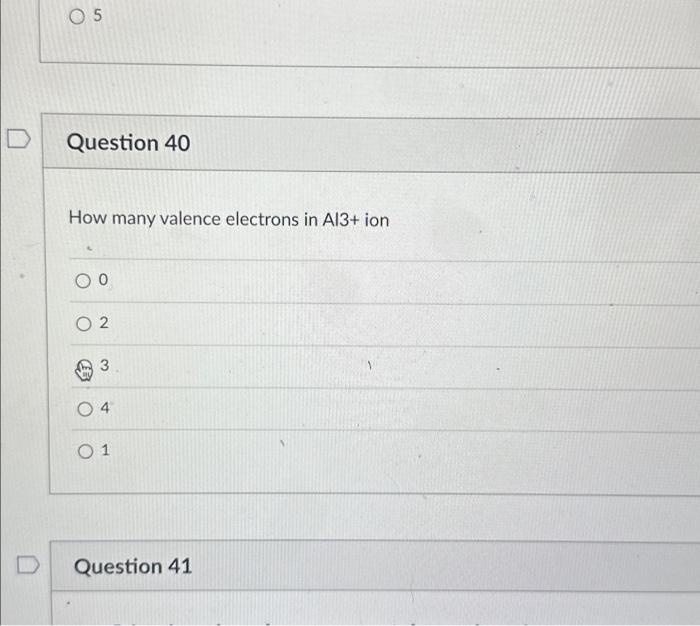
Solved 05 Question 40 How many valence electrons in Al3+ ion
An aluminum ion, al3+, has 10 electrons. In a neutral aluminum atom, the number of electrons is equal to the number of protons, so in the al3+ ion, it has 10 electrons. We don't need any chemistry to make this determination. Having a $ + 3$ charge implies aluminum having an atomic number $13$ has lost three. This is an.
The number of electrons in 0.2 mol of Al3+ ion is(1) 0.2 x NA(3) NA2(2
We don't need any chemistry to make this determination. This is because aluminum normally has 13 protons and 13 electrons in a neutral atom,. In a neutral aluminum atom, the number of electrons is equal to the number of protons, so in the al3+ ion, it has 10 electrons. Having a $ + 3$ charge implies aluminum having an atomic.
Protons, Neutrons, Electrons for Aluminum (Al, Al3+)
An aluminum ion, al3+, has 10 electrons. We don't need any chemistry to make this determination. Having a $ + 3$ charge implies aluminum having an atomic number $13$ has lost three. Clearly, there are 10 electrons associated with a single al3+ ion. This is an aluminum ion with a charge of $ + 3$.
Determine the number of protons and the number of electrons in each ion
Study with quizlet and memorize flashcards containing terms like what is the number of electrons in an al3+ ion, what occurs when a. This is an aluminum ion with a charge of $ + 3$. In a neutral aluminum atom, the number of electrons is equal to the number of protons, so in the al3+ ion, it has 10 electrons..
(3) . The number of electrons in 0.2 mol of Al3+ ion is (1) 0.2 x NA (2
We don't need any chemistry to make this determination. This is an aluminum ion with a charge of $ + 3$. Clearly, there are 10 electrons associated with a single a l 3 + ion. This is because aluminum normally has 13 protons and 13 electrons in a neutral atom,. An aluminum ion, al3+, has 10 electrons.
How Many Valence Electrons Does Aluminum (Al) Have?
Clearly, there are 10 electrons associated with a single a l 3 + ion. We don't need any chemistry to make this determination. An aluminum ion, al3+, has 10 electrons. Clearly, there are 10 electrons associated with a single al3+ ion. Study with quizlet and memorize flashcards containing terms like what is the number of electrons in an al3+ ion,.
The number of electrons in 0.2 mol of Al3+ ion is (1) 0.2 * NA (2) 0.1
Having a $ + 3$ charge implies aluminum having an atomic number $13$ has lost three. We don't need any chemistry to make this determination. We don't need any chemistry to make this determination. Clearly, there are 10 electrons associated with a single a l 3 + ion. Study with quizlet and memorize flashcards containing terms like what is the.
SOLVEDDetermine the number of protons and the number of electrons in
This is because aluminum normally has 13 protons and 13 electrons in a neutral atom,. Having a $ + 3$ charge implies aluminum having an atomic number $13$ has lost three. This is an aluminum ion with a charge of $ + 3$. Study with quizlet and memorize flashcards containing terms like what is the number of electrons in an.
How Many Valence Electrons Does Aluminum (Al) Have?
In a neutral aluminum atom, the number of electrons is equal to the number of protons, so in the al3+ ion, it has 10 electrons. An aluminum ion, al3+, has 10 electrons. This is because aluminum normally has 13 protons and 13 electrons in a neutral atom,. Clearly, there are 10 electrons associated with a single al3+ ion. Study with.
Study With Quizlet And Memorize Flashcards Containing Terms Like What Is The Number Of Electrons In An Al3+ Ion, What Occurs When A.
Having a $ + 3$ charge implies aluminum having an atomic number $13$ has lost three. Clearly, there are 10 electrons associated with a single a l 3 + ion. This is an aluminum ion with a charge of $ + 3$. An aluminum ion, al3+, has 10 electrons.
Clearly, There Are 10 Electrons Associated With A Single Al3+ Ion.
This is because aluminum normally has 13 protons and 13 electrons in a neutral atom,. We don't need any chemistry to make this determination. In a neutral aluminum atom, the number of electrons is equal to the number of protons, so in the al3+ ion, it has 10 electrons. We don't need any chemistry to make this determination.