What Is The Oxidation Number Of Hg In Hg2Cl2
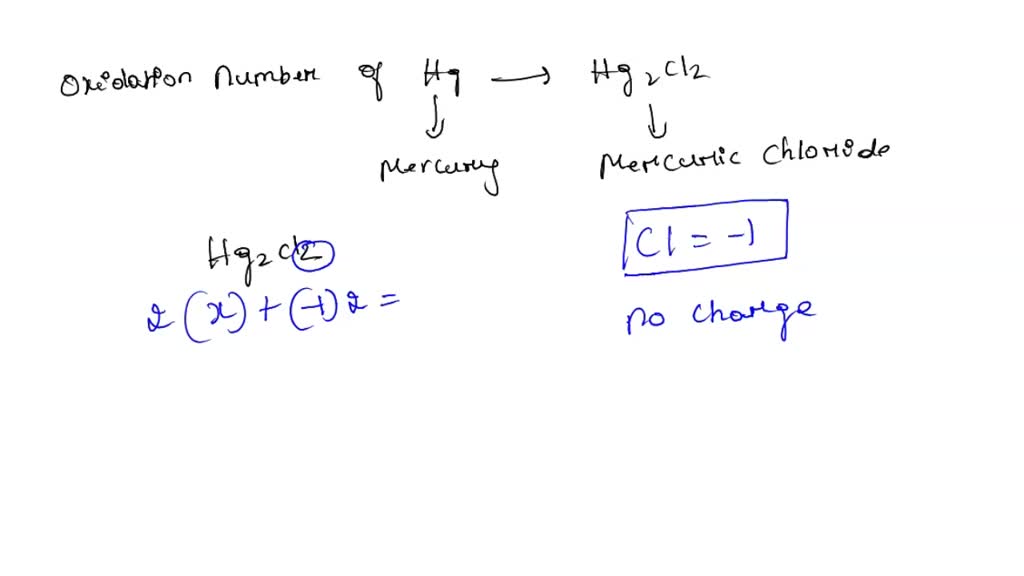
What Is The Oxidation Number Of Hg In Hg2Cl2 - (1) assume the element to be x and since it is hg2 take it 2x. The oxidation number of hg in hg2cl2 is +1. You will look at the oxidation numbers of the reactant and see how it changed in the product. In the case of hg₂cl₂, the oxidation state of mercury is +1. To calculate the oxidation state of hg in hg2cl2. Lets say hg2 for reactants (+1. This can be determined by considering the oxidation states of other.
To calculate the oxidation state of hg in hg2cl2. In the case of hg₂cl₂, the oxidation state of mercury is +1. The oxidation number of hg in hg2cl2 is +1. You will look at the oxidation numbers of the reactant and see how it changed in the product. This can be determined by considering the oxidation states of other. (1) assume the element to be x and since it is hg2 take it 2x. Lets say hg2 for reactants (+1.
In the case of hg₂cl₂, the oxidation state of mercury is +1. (1) assume the element to be x and since it is hg2 take it 2x. Lets say hg2 for reactants (+1. This can be determined by considering the oxidation states of other. To calculate the oxidation state of hg in hg2cl2. The oxidation number of hg in hg2cl2 is +1. You will look at the oxidation numbers of the reactant and see how it changed in the product.
Oxidation number of 'Co' in Hg[Co(SCN)4]
In the case of hg₂cl₂, the oxidation state of mercury is +1. To calculate the oxidation state of hg in hg2cl2. This can be determined by considering the oxidation states of other. (1) assume the element to be x and since it is hg2 take it 2x. The oxidation number of hg in hg2cl2 is +1.
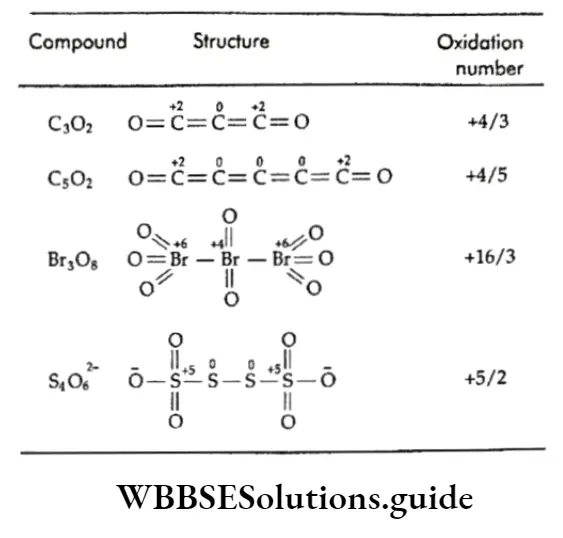
Oxidation Number Definition, Calculation and Examples WBBSE Solutions
To calculate the oxidation state of hg in hg2cl2. You will look at the oxidation numbers of the reactant and see how it changed in the product. (1) assume the element to be x and since it is hg2 take it 2x. This can be determined by considering the oxidation states of other. In the case of hg₂cl₂, the oxidation.
Oxidation Number Rules Chart
In the case of hg₂cl₂, the oxidation state of mercury is +1. You will look at the oxidation numbers of the reactant and see how it changed in the product. The oxidation number of hg in hg2cl2 is +1. To calculate the oxidation state of hg in hg2cl2. This can be determined by considering the oxidation states of other.
Hg oxidation with Cl 2 in N 2 and CO 2 . Download Scientific Diagram
You will look at the oxidation numbers of the reactant and see how it changed in the product. To calculate the oxidation state of hg in hg2cl2. Lets say hg2 for reactants (+1. In the case of hg₂cl₂, the oxidation state of mercury is +1. (1) assume the element to be x and since it is hg2 take it 2x.
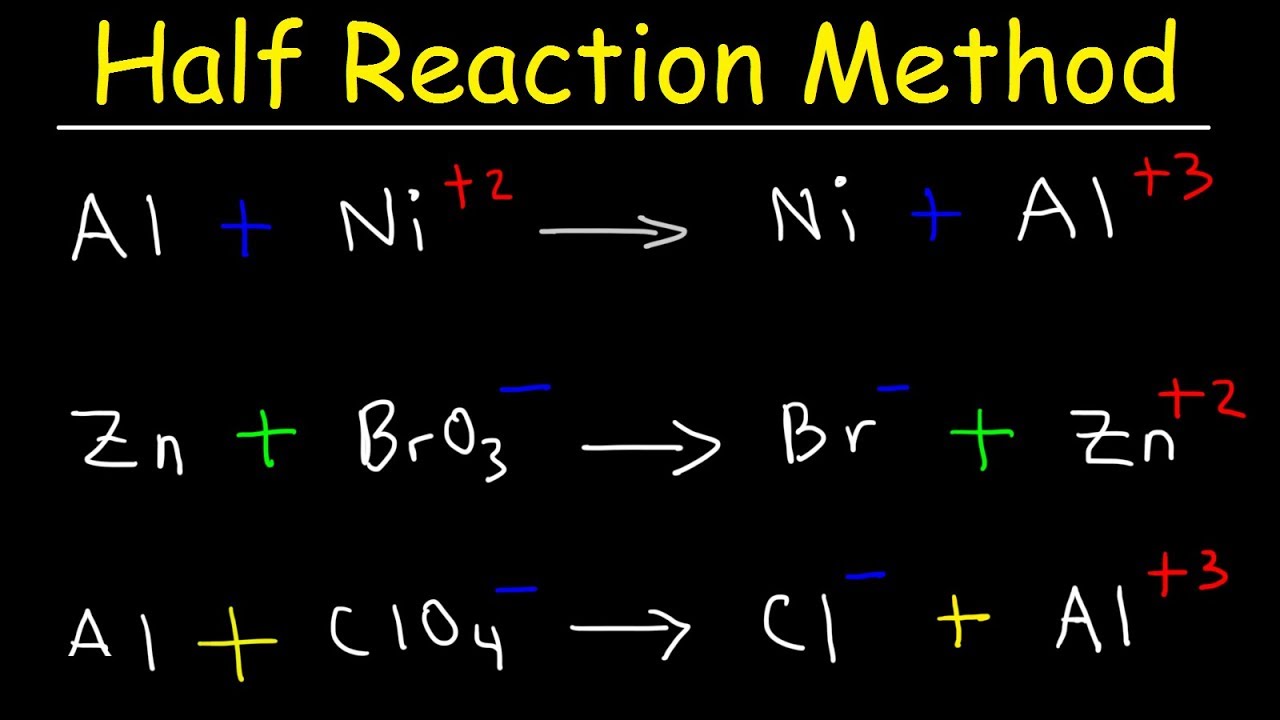
How To Balance A Chemical Equation By Oxidation Number Method In Tamil
The oxidation number of hg in hg2cl2 is +1. In the case of hg₂cl₂, the oxidation state of mercury is +1. This can be determined by considering the oxidation states of other. (1) assume the element to be x and since it is hg2 take it 2x. You will look at the oxidation numbers of the reactant and see how.
Plot of Observed Hg Oxidation Versus Predicted Hg Oxidation, Using
You will look at the oxidation numbers of the reactant and see how it changed in the product. In the case of hg₂cl₂, the oxidation state of mercury is +1. (1) assume the element to be x and since it is hg2 take it 2x. This can be determined by considering the oxidation states of other. To calculate the oxidation.
OXIDATION NUMBER PPT PPT Free Download
(1) assume the element to be x and since it is hg2 take it 2x. In the case of hg₂cl₂, the oxidation state of mercury is +1. You will look at the oxidation numbers of the reactant and see how it changed in the product. The oxidation number of hg in hg2cl2 is +1. Lets say hg2 for reactants (+1.
SOLVED What is the oxidation number of Hg in Hg2Cl2? a) 2 b) 0 c) 1
Lets say hg2 for reactants (+1. This can be determined by considering the oxidation states of other. In the case of hg₂cl₂, the oxidation state of mercury is +1. You will look at the oxidation numbers of the reactant and see how it changed in the product. (1) assume the element to be x and since it is hg2 take.
Rate of Hg 0 photocatalytic oxidation vs. inlet Hg 0 concentration
The oxidation number of hg in hg2cl2 is +1. To calculate the oxidation state of hg in hg2cl2. This can be determined by considering the oxidation states of other. In the case of hg₂cl₂, the oxidation state of mercury is +1. (1) assume the element to be x and since it is hg2 take it 2x.
Oxidation number of chlorine atoms in CaOCl2 are Filo
The oxidation number of hg in hg2cl2 is +1. This can be determined by considering the oxidation states of other. To calculate the oxidation state of hg in hg2cl2. In the case of hg₂cl₂, the oxidation state of mercury is +1. (1) assume the element to be x and since it is hg2 take it 2x.
This Can Be Determined By Considering The Oxidation States Of Other.
Lets say hg2 for reactants (+1. In the case of hg₂cl₂, the oxidation state of mercury is +1. (1) assume the element to be x and since it is hg2 take it 2x. You will look at the oxidation numbers of the reactant and see how it changed in the product.
To Calculate The Oxidation State Of Hg In Hg2Cl2.
The oxidation number of hg in hg2cl2 is +1.
![Oxidation number of 'Co' in Hg[Co(SCN)4]](https://d1hj4to4g9ba46.cloudfront.net/questions/2021628_1318215_ans_11fe87bacafb4aefbff9e7364e9f75f0.jpg)