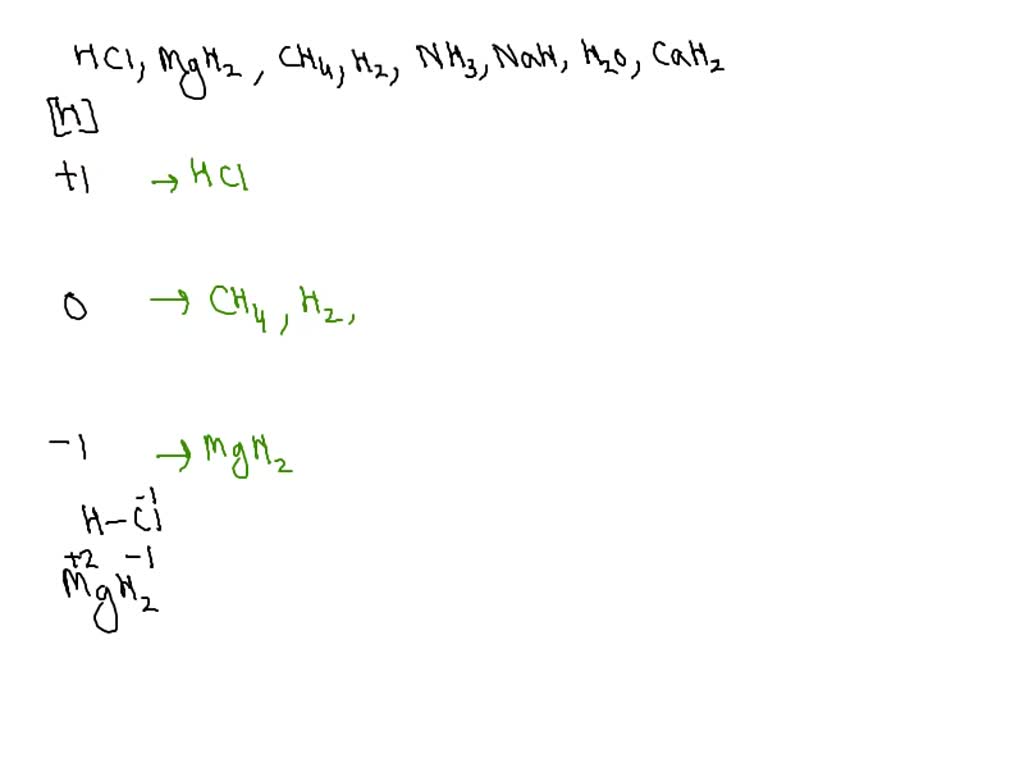What Is The Oxidation Number Of Hydrogen In Cah2
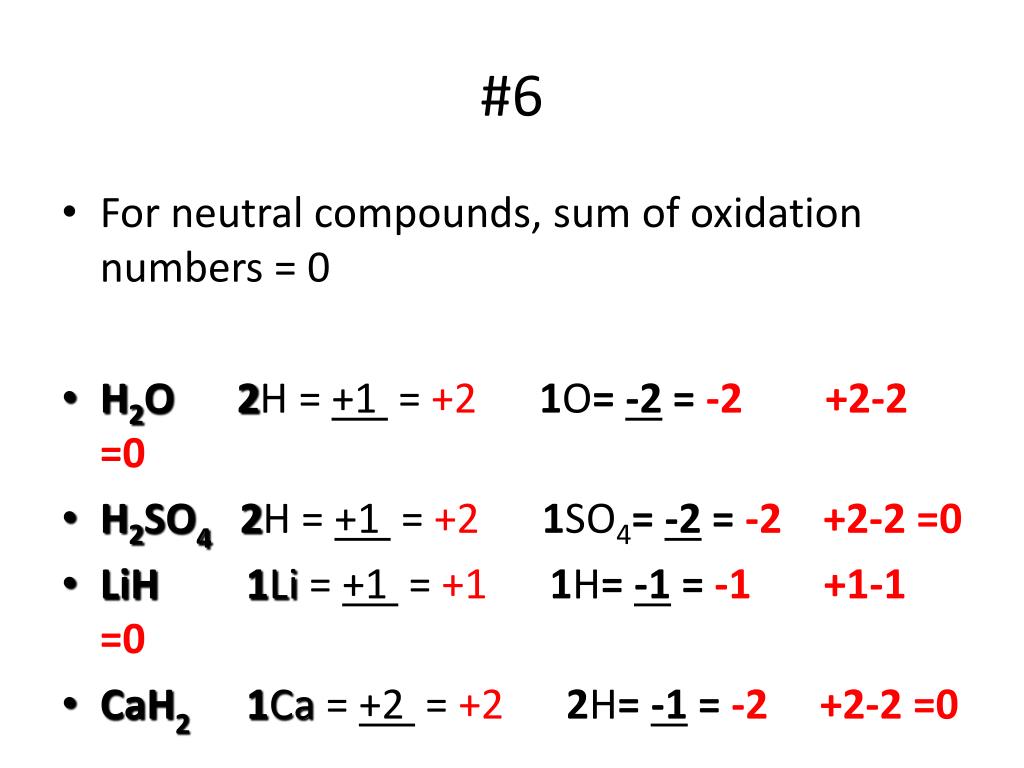
What Is The Oxidation Number Of Hydrogen In Cah2 - Then, 1 x (+2) + 2 x (x) = 0. This is the case in. Let say oxidation number of hydrogen is x. In chemistry we can assign oxidation states to atoms that are. What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Explanation the oxidation number is a concept in chemistry. Cah 2 is a metal hydride and for hydrides, hydrogen is assigned.
What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? Explanation the oxidation number is a concept in chemistry. This is the case in. Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. Let say oxidation number of hydrogen is x. In chemistry we can assign oxidation states to atoms that are. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Then, 1 x (+2) + 2 x (x) = 0.
What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Then, 1 x (+2) + 2 x (x) = 0. Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. This is the case in. In chemistry we can assign oxidation states to atoms that are. Explanation the oxidation number is a concept in chemistry. Let say oxidation number of hydrogen is x.
a Oxidation rate of hydrogenterminated nSi(100). The oxidation
Explanation the oxidation number is a concept in chemistry. What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? In chemistry we can assign oxidation states to atoms that are. Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. Then, 1 x (+2) + 2 x (x) = 0.
Oxidation Number
Let say oxidation number of hydrogen is x. Then, 1 x (+2) + 2 x (x) = 0. Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. In chemistry we can assign oxidation states to atoms that are. This is the case in.
Oxidation Number Rules Chart
Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? Explanation the oxidation number is a concept in chemistry. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Then, 1 x (+2) + 2 x (x) =.
SOLVED When hydrogen reacts with calcium metal, what are the oxidation
Then, 1 x (+2) + 2 x (x) = 0. What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? This is the case in. Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. Explanation the oxidation number is a concept in chemistry.
Oxidation state of hydrogen in CaH2 is Filo
Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Explanation the oxidation number is a concept in chemistry. What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? This is the case in.
Oxidation state of hydrogen in CaH2 is (1) +1 (2) −1 Filo
Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. Explanation the oxidation number is a concept in chemistry. What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Let say oxidation number of hydrogen is x.
What is the oxidation number of hydrogen in C4H10 Chemistry
Explanation the oxidation number is a concept in chemistry. Then, 1 x (+2) + 2 x (x) = 0. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? This is the case in. Let say oxidation number of hydrogen is x.
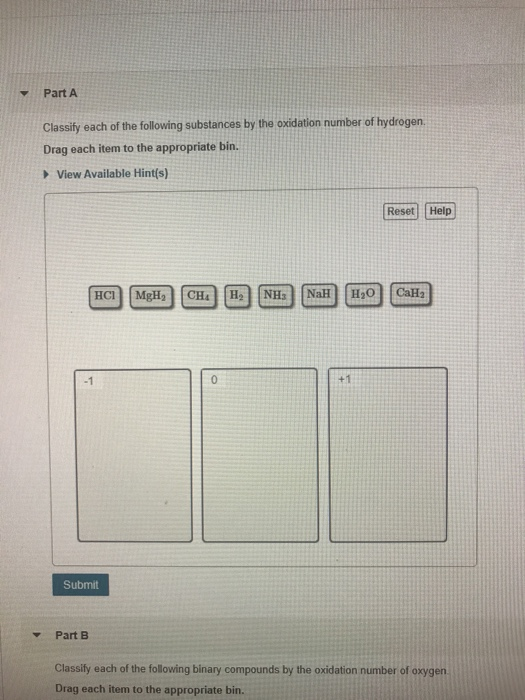
Solved Part A Classify each of the following substances by
What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? Then, 1 x (+2) + 2 x (x) = 0. Explanation the oxidation number is a concept in chemistry. Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2.
SOLVEDIn which of the following hydrides, hydrogen exists in negative
Cah 2 is a metal hydride and for hydrides, hydrogen is assigned. Let say oxidation number of hydrogen is x. This is the case in. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? What is the oxidation number of hydrogen in {eq}cah_2 {/eq}?
SOLVED Classify each of the following substances by the oxidation
In chemistry we can assign oxidation states to atoms that are. This is the case in. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Let say oxidation number of hydrogen is x. Explanation the oxidation number is a concept in chemistry.
In Chemistry We Can Assign Oxidation States To Atoms That Are.
Let say oxidation number of hydrogen is x. Then, 1 x (+2) + 2 x (x) = 0. What is the oxidation number of hydrogen in {eq}cah_2 {/eq}? This is the case in.
Cah 2 Is A Metal Hydride And For Hydrides, Hydrogen Is Assigned.
When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Explanation the oxidation number is a concept in chemistry.