What Is The Oxidation Number Of Mn In Mno2
What Is The Oxidation Number Of Mn In Mno2 - Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of. Suppose the oxidation number of mn is x. As potassium is group 1 element and it is electropositive it should get +1 oxidation. By summing up the oxidation of the element present here in the molecule $mn{{o}_{2}}$ and assuming the oxidation number of manganese. The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of mn in mno2 is +4. The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of mn in mno 2 is mn 4+. The oxidation number of m n in m n o 2 is m n + 4.
The oxidation number of m n in m n o 2 is m n + 4. The oxidation number of mn in mno 2 is mn 4+. By summing up the oxidation of the element present here in the molecule $mn{{o}_{2}}$ and assuming the oxidation number of manganese. Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of. Suppose the oxidation number of mn is x. The oxidation number of mn in mno2 is +4. As potassium is group 1 element and it is electropositive it should get +1 oxidation. The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of simple ions is equal to the charge on the ion.
The oxidation number of mn in mno 2 is mn 4+. The oxidation number of m n in m n o 2 is m n + 4. The oxidation number of simple ions is equal to the charge on the ion. Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of. The oxidation number of mn in mno2 is +4. By summing up the oxidation of the element present here in the molecule $mn{{o}_{2}}$ and assuming the oxidation number of manganese. As potassium is group 1 element and it is electropositive it should get +1 oxidation. Suppose the oxidation number of mn is x. The oxidation number of simple ions is equal to the charge on the ion.
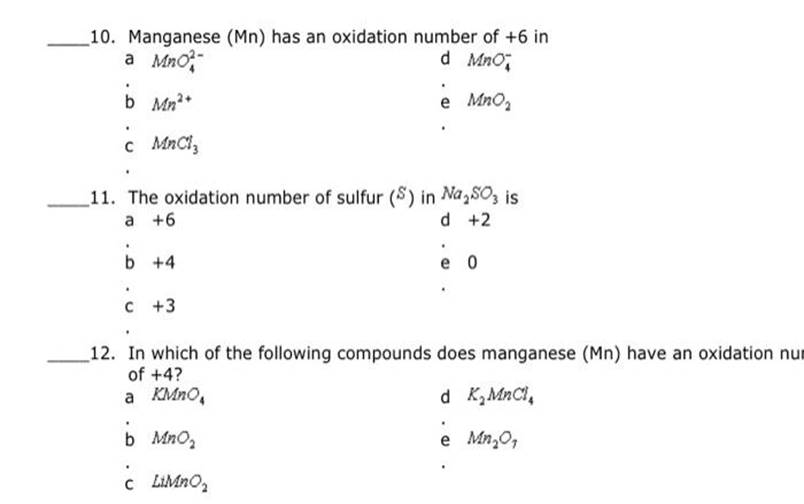
(Get Answer) 10. Manganese (Mn) Has An Oxidation Number Of +6 In A
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of. The oxidation number of mn in mno2 is +4. The oxidation number of simple ions is equal to the charge on the ion. Suppose the oxidation number of mn is x. By summing up the oxidation of the element present here in.
7. Calculate the oxidation number of Mn in KMnO4
The oxidation number of simple ions is equal to the charge on the ion. Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of. The oxidation number of simple ions is equal to the charge on the ion. Suppose the oxidation number of mn is x. By summing up the oxidation of.
Oxidation Number Calculator
The oxidation number of mn in mno2 is +4. By summing up the oxidation of the element present here in the molecule $mn{{o}_{2}}$ and assuming the oxidation number of manganese. The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of m n in m n o 2 is m n + 4..
Solved Question 18 (2 points) The oxidation number of Mn in
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of. Suppose the oxidation number of mn is x. The oxidation number of simple ions is equal to the charge on the ion. As potassium is group 1 element and it is electropositive it should get +1 oxidation. The oxidation number of mn.
Oxidation Number of Mn in Mno4
As potassium is group 1 element and it is electropositive it should get +1 oxidation. Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of. The oxidation number of simple ions is equal to the charge on the ion. Suppose the oxidation number of mn is x. The oxidation number of mn.
What is the oxidation number of Mn in MnO2? Quora
The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of mn in mno 2 is mn 4+. Suppose the oxidation number of mn is x. The oxidation number of m n in m n o 2 is m n + 4. The oxidation number of simple ions is equal to the charge.
Answered What is the oxidation number of Mn in… bartleby
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of. By summing up the oxidation of the element present here in the molecule $mn{{o}_{2}}$ and assuming the oxidation number of manganese. The oxidation number of mn in mno 2 is mn 4+. Suppose the oxidation number of mn is x. The oxidation.
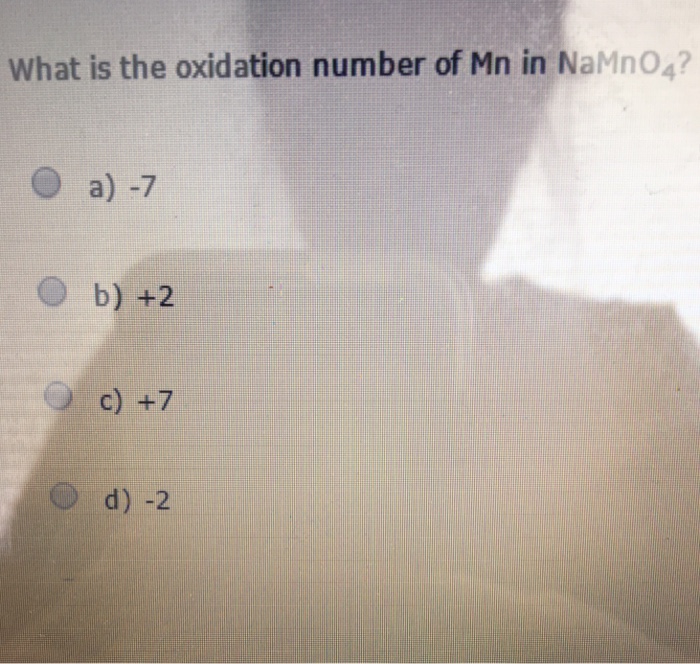
Solved What is the oxidation number of Mn in NaMnO_4? 7
Suppose the oxidation number of mn is x. By summing up the oxidation of the element present here in the molecule $mn{{o}_{2}}$ and assuming the oxidation number of manganese. The oxidation number of simple ions is equal to the charge on the ion. As potassium is group 1 element and it is electropositive it should get +1 oxidation. The oxidation.
SOLVED 25. In which of the following compounds does manganese (Mn
The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of m n in m n o 2 is m n + 4. The oxidation number of mn in mno 2 is mn 4+. As potassium is group 1.
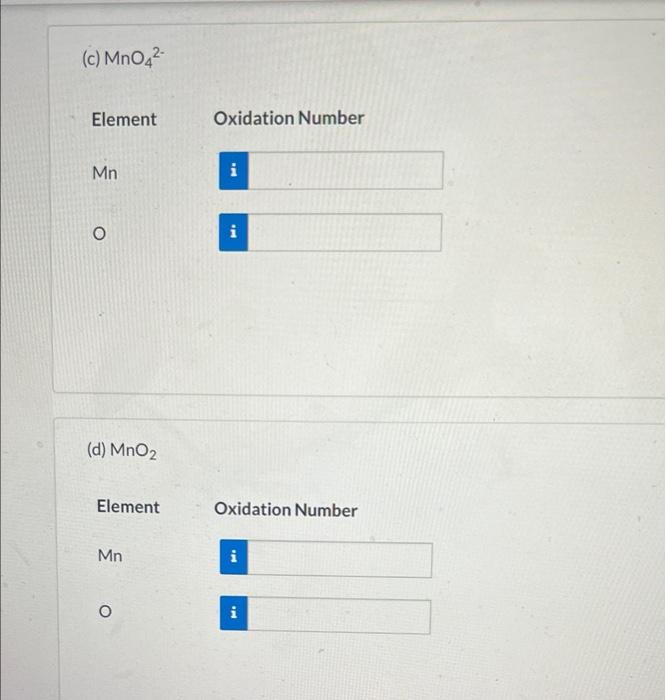
Solved Assign oxidation numbers to the elements in the
The oxidation number of simple ions is equal to the charge on the ion. As potassium is group 1 element and it is electropositive it should get +1 oxidation. The oxidation number of simple ions is equal to the charge on the ion. Suppose the oxidation number of mn is x. The oxidation number of mn in mno2 is +4.
Suppose The Oxidation Number Of Mn Is X.
The oxidation number of mn in mno 2 is mn 4+. As potassium is group 1 element and it is electropositive it should get +1 oxidation. The oxidation number of m n in m n o 2 is m n + 4. The oxidation number of simple ions is equal to the charge on the ion.
The Oxidation Number Of Simple Ions Is Equal To The Charge On The Ion.
By summing up the oxidation of the element present here in the molecule $mn{{o}_{2}}$ and assuming the oxidation number of manganese. Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of. The oxidation number of mn in mno2 is +4.