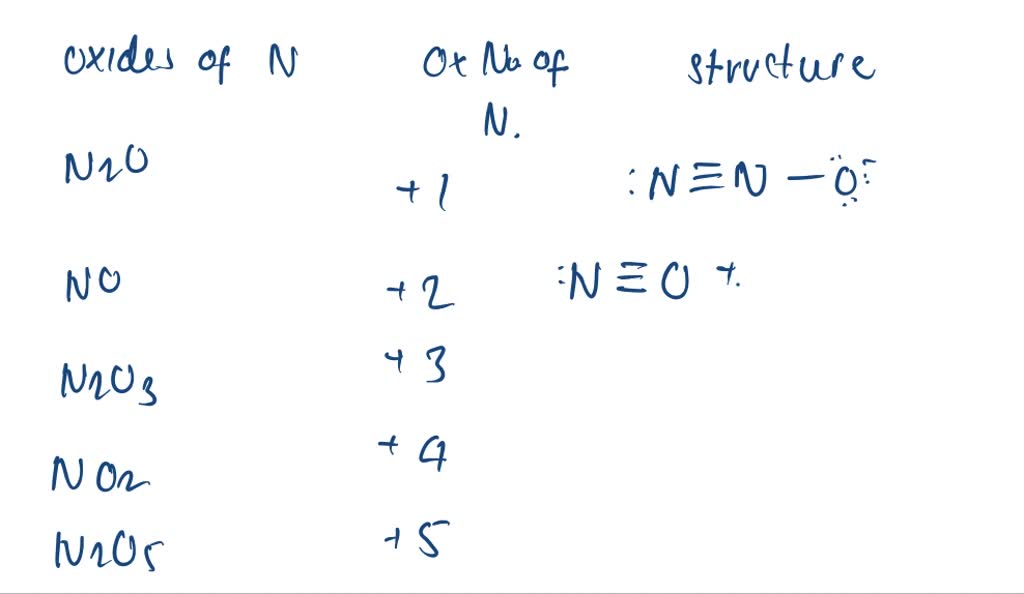What Is The Oxidation Number Of Nitrogen
What Is The Oxidation Number Of Nitrogen - The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Each nitrogen gained one electron,.
Each nitrogen gained one electron,. The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced.
Each nitrogen gained one electron,. The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced.
RR0001 11. Oxidation state 2. The oxidation number of nitrogen in NH2 OH
Each nitrogen gained one electron,. The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced.
Oxidation Number (State) Definition, Rules, How to Find, and Examples
The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Each nitrogen gained one electron,.
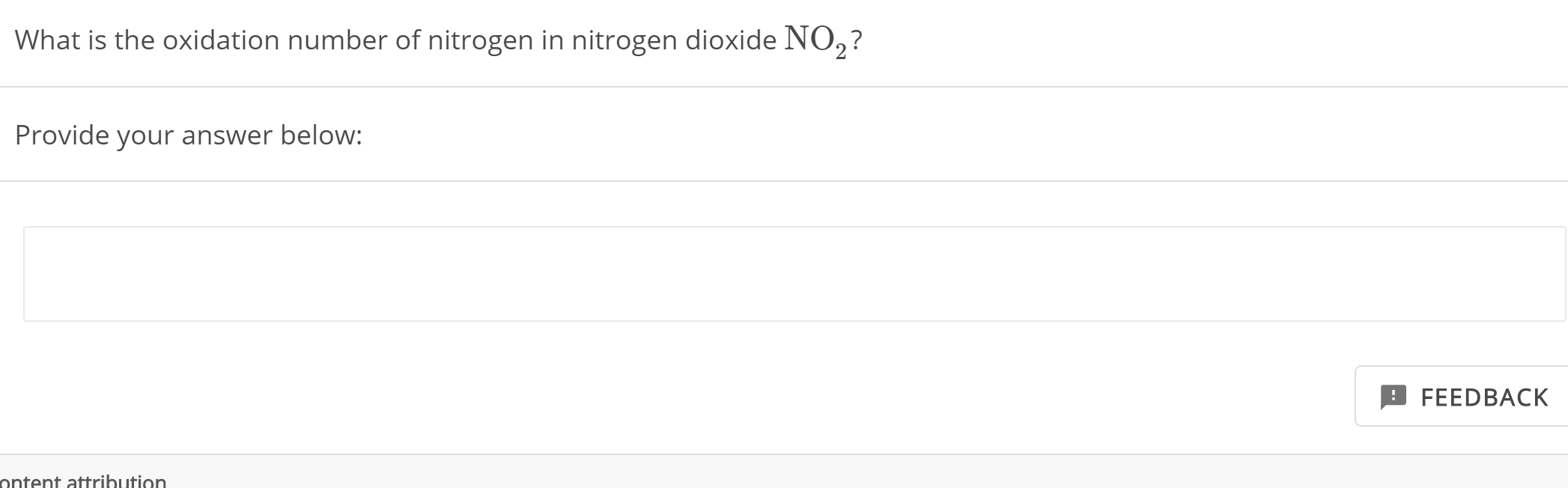
Solved What is the oxidation number of nitrogen in nitrogen
Each nitrogen gained one electron,. The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced.
SOLVEDList the different nitrogen oxides. What is the oxidation number
Each nitrogen gained one electron,. The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced.
What Is the Highest Possible Oxidation Number of Nitrogen? Sciencing
Each nitrogen gained one electron,. The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced.
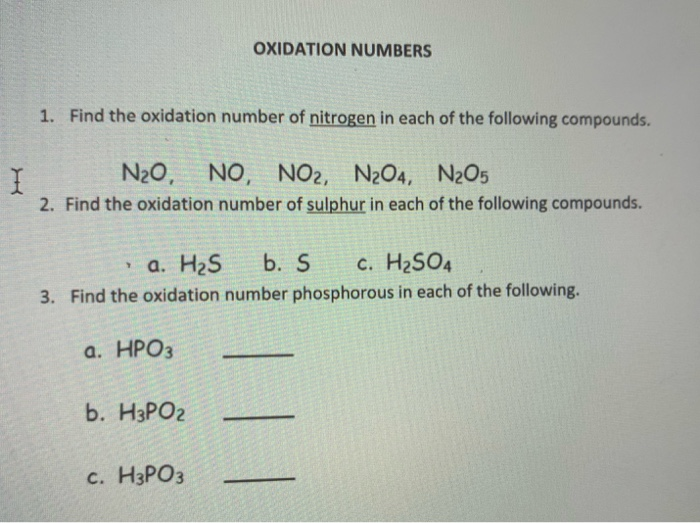
Solved OXIDATION NUMBERS 1. Find the oxidation number of
The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Each nitrogen gained one electron,.
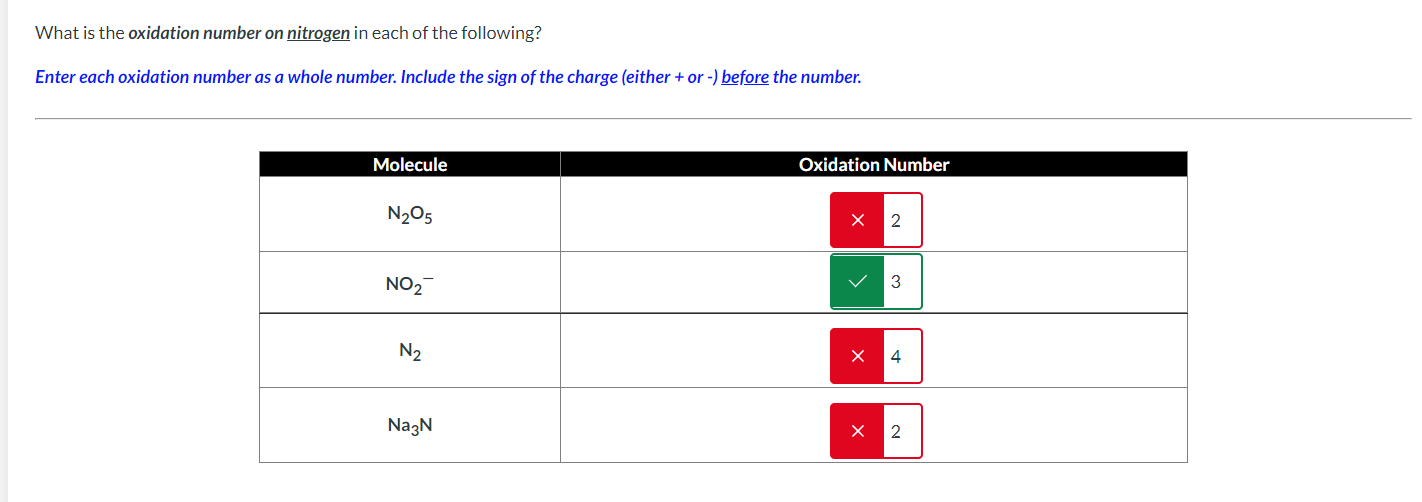
Solved What is the oxidation number on nitrogen in each of
The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Each nitrogen gained one electron,.
Oxidation states of nitrogen MEL Chemistry
The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Each nitrogen gained one electron,.
Oxidation Number of Nitrogen ManueltaroHuerta
Each nitrogen gained one electron,. The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced.
Each Nitrogen Gained One Electron,.
The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced.