What Is The Oxidation State Of Cl In Clo2
What Is The Oxidation State Of Cl In Clo2 - In clo2, the oxygen atom is. So the oxidation number of c l. Oxygen usually has an oxidation number of − 2. The oxidation state of chlorine in clo2 is +4. We put the oxidation state and equate it equal to zero. The oxidation state of cl in clo2 is +3. The oxidation states of cl are: Recall the rules for assigning oxidation. For two o atoms, the total oxidation number is − 4. So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4.
So the oxidation number of c l. The oxidation state of chlorine in clo2 is +4. The oxidation states of cl are: Recall the rules for assigning oxidation. Oxygen usually has an oxidation number of − 2. So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. We put the oxidation state and equate it equal to zero. In clo2, the oxygen atom is. For two o atoms, the total oxidation number is − 4. Here's a brief explanation of how to determine this:
So the oxidation number of c l. In clo2, the oxygen atom is. Recall the rules for assigning oxidation. Here's a brief explanation of how to determine this: The oxidation state of cl in clo2 is +3. The oxidation state of chlorine in clo2 is +4. The oxidation states of cl are: So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. For two o atoms, the total oxidation number is − 4. Oxygen usually has an oxidation number of − 2.
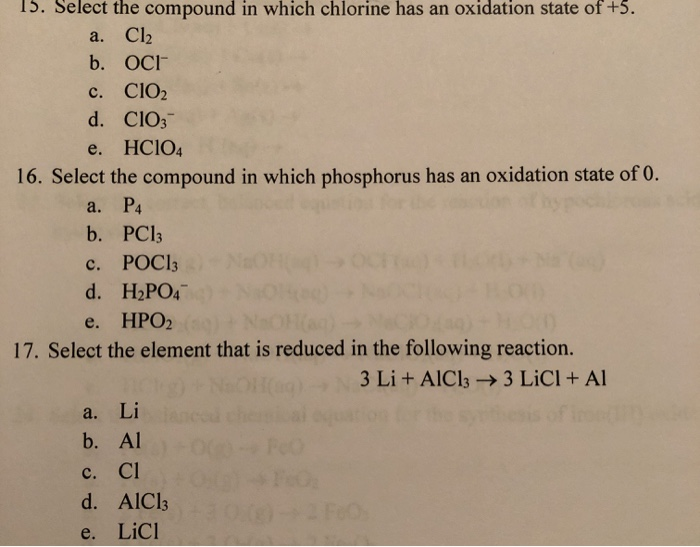
Solved 15. Select the compound in which chlorine has an
In clo2, the oxygen atom is. The oxidation state of cl in clo2 is +3. So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. Recall the rules for assigning oxidation. So the oxidation number of c l.
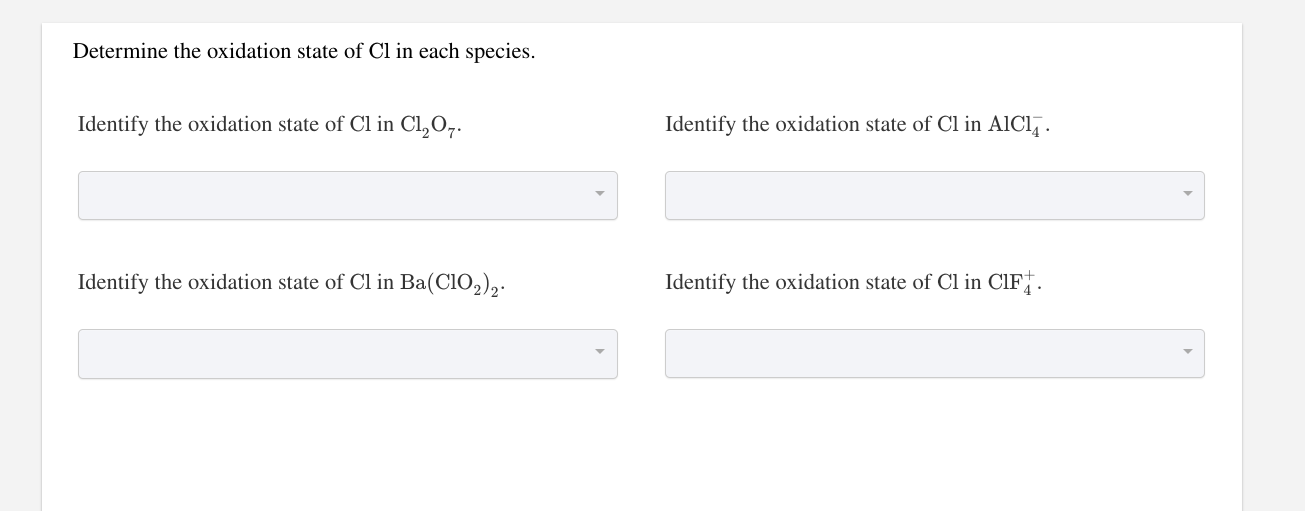
Solved Determine the oxidation state of Cl in each species.
So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. In clo2, the oxygen atom is. Oxygen usually has an oxidation number of − 2. The oxidation states of cl are: So the oxidation number of c l.
[Solved] Determine the oxidation state of Cl Cl in each species
Here's a brief explanation of how to determine this: We put the oxidation state and equate it equal to zero. The oxidation state of cl in clo2 is +3. So the oxidation number of c l. For two o atoms, the total oxidation number is − 4.
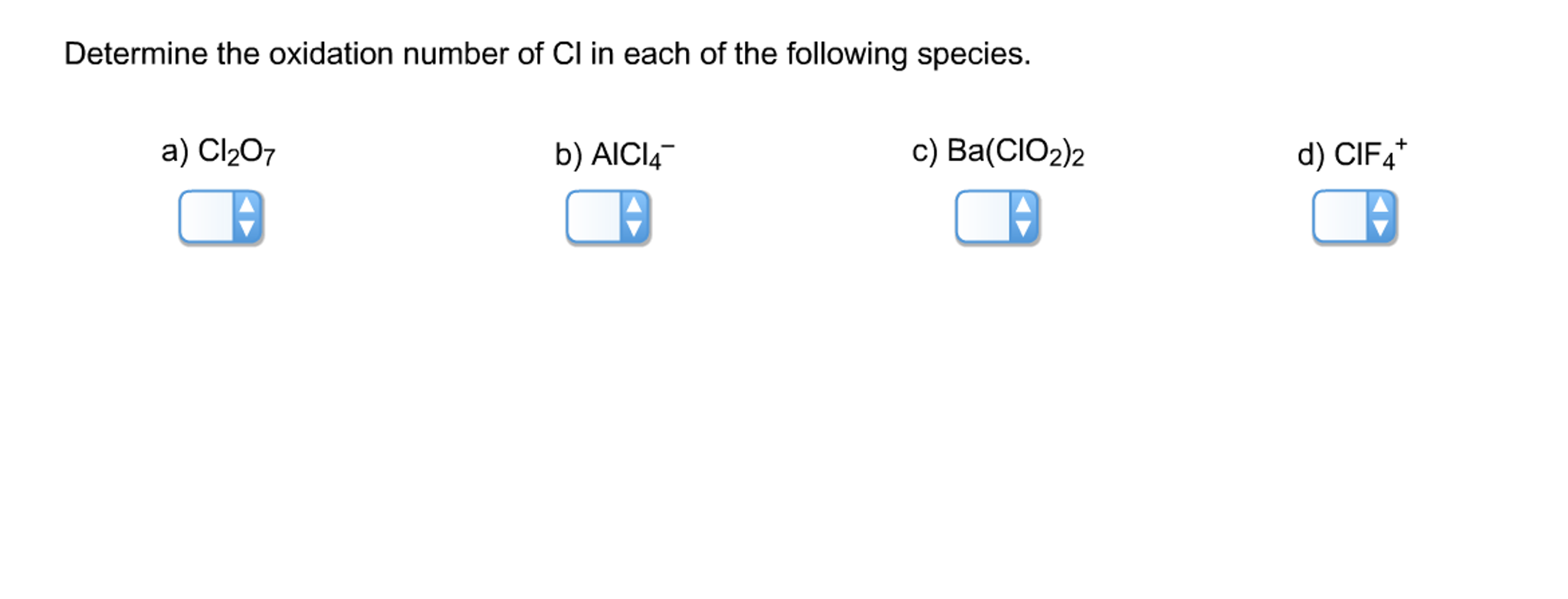
Solved Determine the oxidation number of Cl in each of the
Oxygen usually has an oxidation number of − 2. The oxidation state of cl in clo2 is +3. In clo2, the oxygen atom is. So the oxidation number of c l. The oxidation states of cl are:
oxidation number of Both Cl in Ca(OCl)Cl
Here's a brief explanation of how to determine this: We put the oxidation state and equate it equal to zero. So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. In clo2, the oxygen atom is. Recall the rules for assigning oxidation.
What is the oxidation number of Cl in CaOCl2 Explain with structure
Recall the rules for assigning oxidation. So the oxidation number of c l. In clo2, the oxygen atom is. So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. Here's a brief explanation of how to determine this:
42. How to find oxidation state of central atom maximum oxidation state
The oxidation states of cl are: So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. Oxygen usually has an oxidation number of − 2. So the oxidation number of c l. We put the oxidation state and equate it equal to zero.
What is the oxidation state of chlorine and oxygen in Cl2O6 and Cl2O7
So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. So the oxidation number of c l. For two o atoms, the total oxidation number is − 4. Oxygen usually has an oxidation number of − 2. Recall the rules for assigning oxidation.
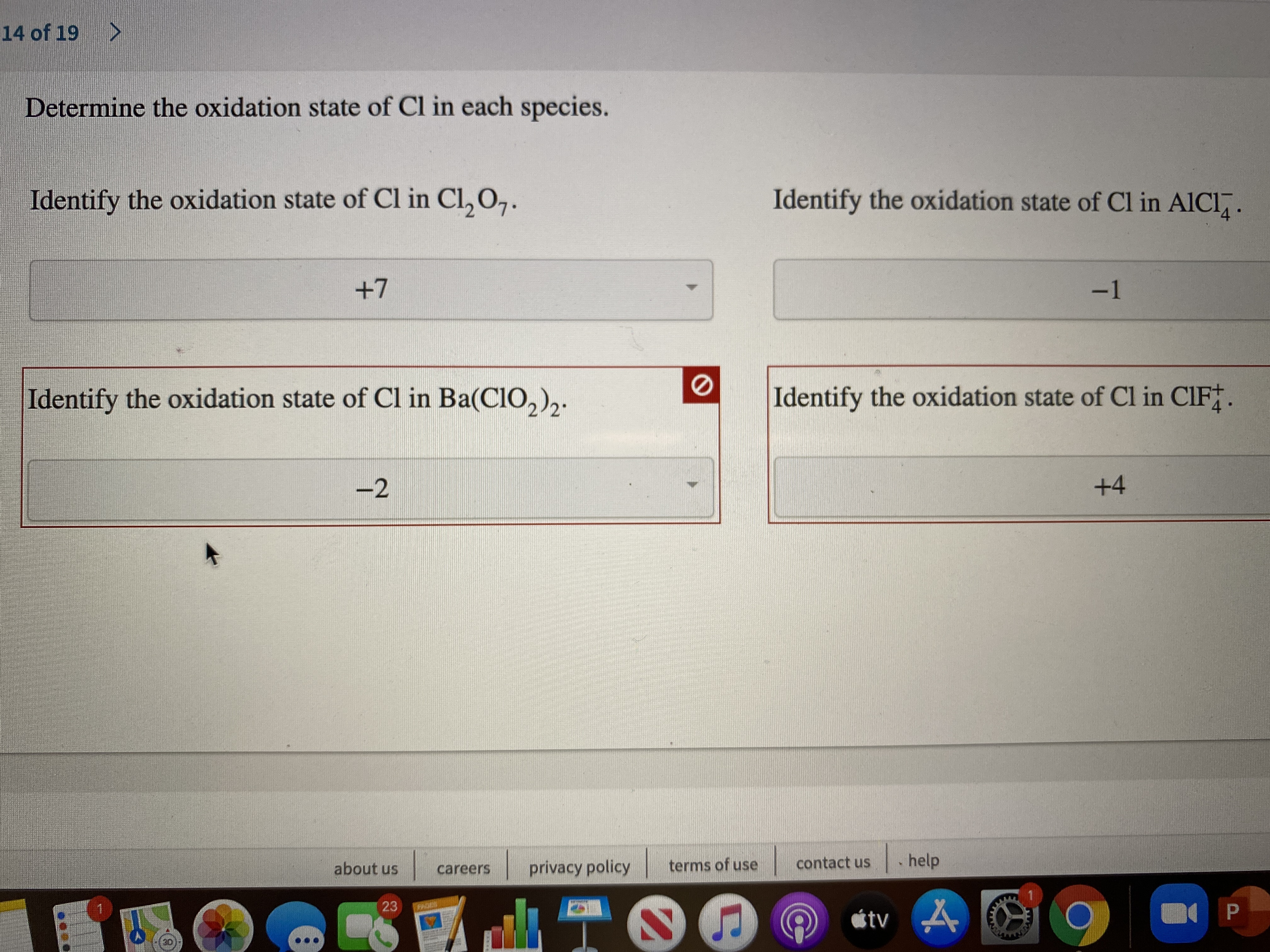
Answered Determine the oxidation state of Cl in… bartleby
The oxidation states of cl are: The oxidation state of chlorine in clo2 is +4. Here's a brief explanation of how to determine this: Recall the rules for assigning oxidation. We put the oxidation state and equate it equal to zero.
For Two O Atoms, The Total Oxidation Number Is − 4.
The oxidation states of cl are: Recall the rules for assigning oxidation. Here's a brief explanation of how to determine this: So the oxidation number of c l.
So, We Had Calculated The Oxidation State Of Chlorine In $Cl {O_2}$ Is 4.
In clo2, the oxygen atom is. Oxygen usually has an oxidation number of − 2. The oxidation state of chlorine in clo2 is +4. We put the oxidation state and equate it equal to zero.