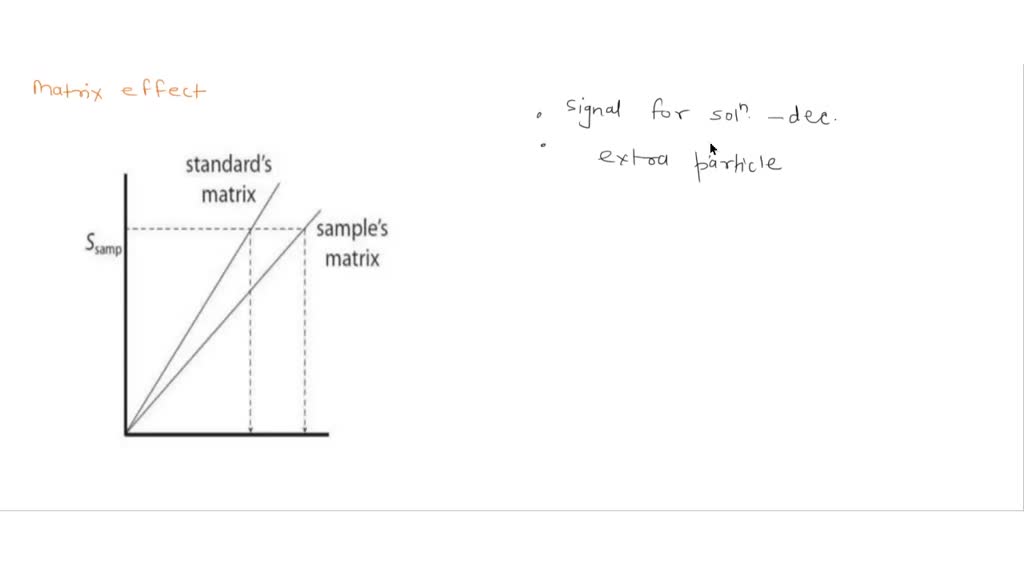
What Is The Oxidation State Of Sulfur In Na2S2O3
What Is The Oxidation State Of Sulfur In Na2S2O3 - Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. It contains two types of sulfur. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal The equivalent weight of na2s2o3 (mol. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns.
Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal The equivalent weight of na2s2o3 (mol. It contains two types of sulfur. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns.
It contains two types of sulfur. Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal The equivalent weight of na2s2o3 (mol. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns.
Solved 2. Determine the oxidation state of sulfur in S8,
The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. The equivalent weight of na2s2o3 (mol. It contains two types of sulfur.
Solved Determine the oxidation state for each of the
Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal The equivalent weight of na2s2o3 (mol. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. It contains two types of sulfur. Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate.
Periodic Table Sulfur Oxidation Number Periodic Table Timeline
Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. The equivalent weight of na2s2o3 (mol. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal It contains two types of sulfur.
What is the oxidation state of sulfur in the following (b) SCl2
It contains two types of sulfur. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal The equivalent weight of na2s2o3 (mol. Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate.
SOLVED 53. In which of the following compounds does sulfur exist in
Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. The equivalent weight of na2s2o3 (mol. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. It contains two types of sulfur. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal
Why is the oxidation state in sulfur +5 and not +6? r/Mcat
It contains two types of sulfur. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. The equivalent weight of na2s2o3 (mol. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal
Sulfur oxidation steps related to temperature Download Table
The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. It contains two types of sulfur. The equivalent weight of na2s2o3 (mol. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal
[ANSWERED] Determine the oxidation state of sulfur i... Physical
It contains two types of sulfur. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. The equivalent weight of na2s2o3 (mol.
⏩SOLVEDWhat is the oxidation state of sulfur in the following… Numerade
Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal It contains two types of sulfur. The equivalent weight of na2s2o3 (mol.
SOLVED The oxidation number for sulfur in the compound sodium
Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. Wt = m) in the reaction 2na2s2o3 + i2 → na2s4o6 + 2 nal The equivalent weight of na2s2o3 (mol. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns. It contains two types of sulfur.
Wt = M) In The Reaction 2Na2S2O3 + I2 → Na2S4O6 + 2 Nal
It contains two types of sulfur. The equivalent weight of na2s2o3 (mol. Oxidation state of sulfur in na2s2o3 the compound na2s2o3 is also known as sodium thiosulfate. The oxidation state of sulfur in na2s2o3 (sodium thiosulfate) is determined by finding the average oxidation state, which turns.



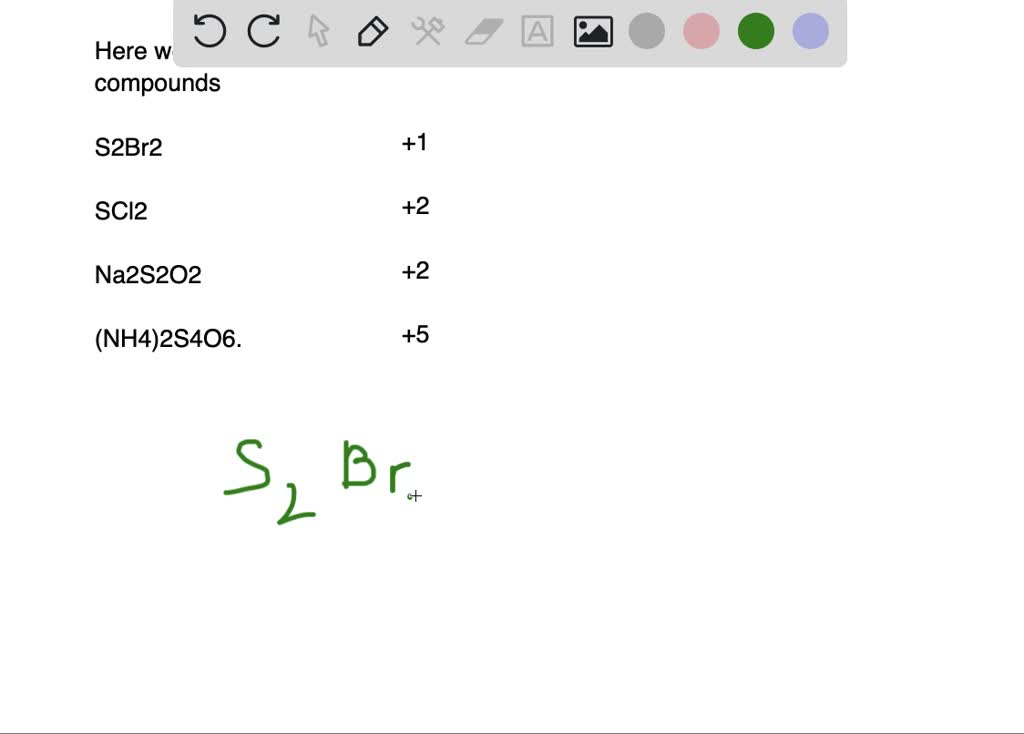
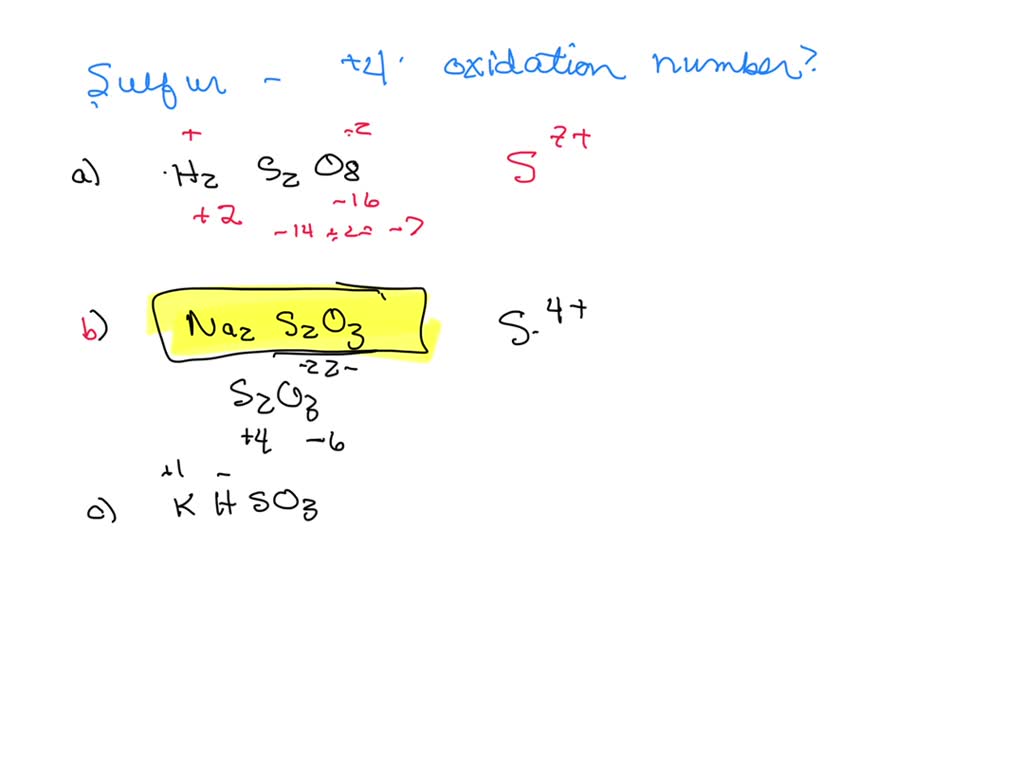


![[ANSWERED] Determine the oxidation state of sulfur i... Physical](https://media.kunduz.com/media/sug-question/raw/77081382-1659002929.5740907.jpeg?h=512)