What Is The Percent Composition Of Sulfur In H2So4
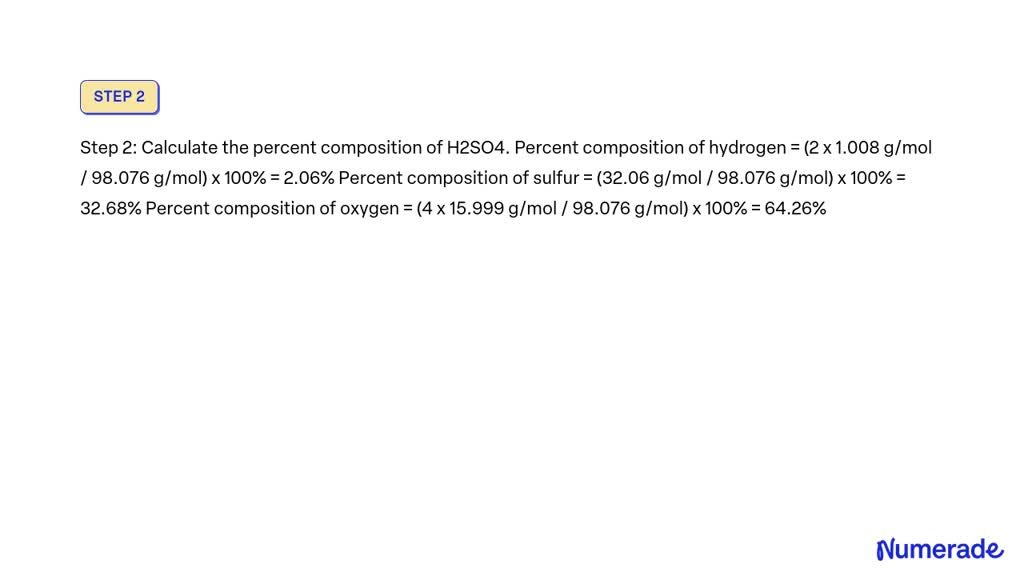
What Is The Percent Composition Of Sulfur In H2So4 - The molar mass of sulfur (s) is 32.06 g/mol, and the molar mass of h2so4 is 98.09 g/mol. The percent composition of sulfur in sulfuric acid (h2so4) is 32.7%. 32.7% explanation to determine the percent composition of sulfur in sulfuric acid. The percent composition of sulfur in h2so4 is 32.7%. % composition = ( mass s / mass h2so4 ) × 100 = 32.08/ 98.10 × 100 =. This is found by dividing the molar mass of sulfur by the total. The question is asking for the percent composition of sulfur in the chemical. The percent composition of sulfur in h₂so₄ is: Log in for more information. What is the percent composition of sulfur in h2so4?
This answer has been confirmed as correct and. The percent composition of sulfur in h2so4 is 32.7%. The percent composition of sulfur in sulfuric acid (h₂so₄) is approximately 32.65%, which aligns closely with option a. The percent composition of sulfur in sulfuric acid (h2so4) is 32.7%. This is found by dividing the molar mass of sulfur by the total. What is the percent composition of sulfur in h2so4? Log in for more information. 32.7% explanation to determine the percent composition of sulfur in sulfuric acid. The percent composition of sulfur in h₂so₄ is: The molar mass of sulfur (s) is 32.06 g/mol, and the molar mass of h2so4 is 98.09 g/mol.
What is the percent composition of sulfur in h2so4? The percent composition of sulfur in sulfuric acid (h2so4) is 32.7%. The molar mass of sulfur (s) is 32.06 g/mol, and the molar mass of h2so4 is 98.09 g/mol. The question is asking for the percent composition of sulfur in the chemical. % composition = ( mass s / mass h2so4 ) × 100 = 32.08/ 98.10 × 100 =. The percent composition of sulfur in h₂so₄ is: This is found by dividing the molar mass of sulfur by the total. This answer has been confirmed as correct and. 32.7% explanation to determine the percent composition of sulfur in sulfuric acid. The percent composition of sulfur in h2so4 is 32.7%.
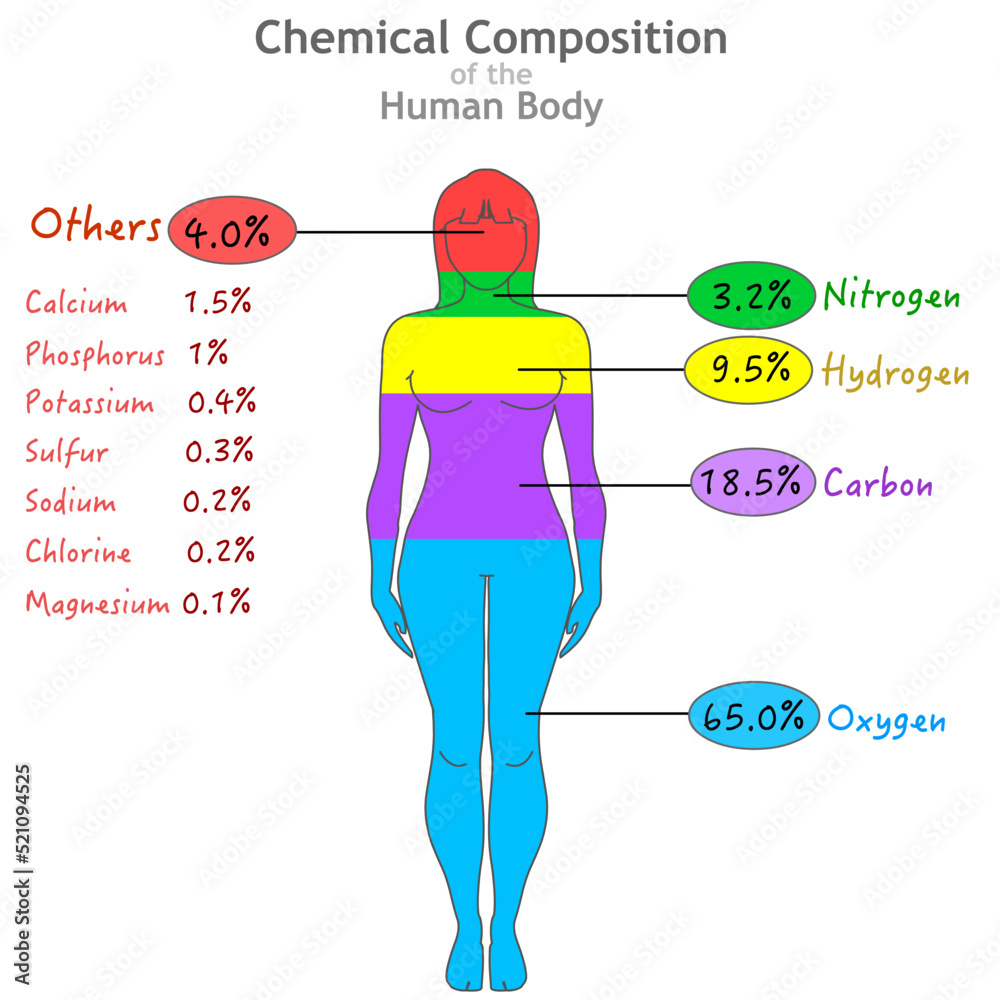
Chemical composition. Elements in human body. Percent ratios of oxygen
The percent composition of sulfur in h2so4 is 32.7%. The percent composition of sulfur in h₂so₄ is: What is the percent composition of sulfur in h2so4? The molar mass of sulfur (s) is 32.06 g/mol, and the molar mass of h2so4 is 98.09 g/mol. This is found by dividing the molar mass of sulfur by the total.
Sulfur chemical Element Graphic for Science Designs Stock Vector Image
This is found by dividing the molar mass of sulfur by the total. This answer has been confirmed as correct and. The molar mass of sulfur (s) is 32.06 g/mol, and the molar mass of h2so4 is 98.09 g/mol. 32.7% explanation to determine the percent composition of sulfur in sulfuric acid. 32.7% is the percent composition of sulfur in h2so4.
SOLVED Calculate the percent by mass sulfur in sulfuric acid, H2SO4.
% composition = ( mass s / mass h2so4 ) × 100 = 32.08/ 98.10 × 100 =. Log in for more information. 32.7% explanation to determine the percent composition of sulfur in sulfuric acid. The percent composition of sulfur in sulfuric acid (h₂so₄) is approximately 32.65%, which aligns closely with option a. The percent composition of sulfur in h2so4.
Solved The percentage mass of sulfur ( S ) in H2SO4 isUse
The percent composition of sulfur in h2so4 is 32.7%. 32.7% is the percent composition of sulfur in h2so4. Log in for more information. This answer has been confirmed as correct and. The percent composition of sulfur in sulfuric acid (h₂so₄) is approximately 32.65%, which aligns closely with option a.
Sulfur Or Sulfur Is A Chemical Element Used For Sulfuric Acid For
This answer has been confirmed as correct and. Log in for more information. % composition = ( mass s / mass h2so4 ) × 100 = 32.08/ 98.10 × 100 =. This is found by dividing the molar mass of sulfur by the total. The percent composition of sulfur in sulfuric acid (h2so4) is 32.7%.
SOLVED Determine the percent composition of each element in the
This is found by dividing the molar mass of sulfur by the total. % composition = ( mass s / mass h2so4 ) × 100 = 32.08/ 98.10 × 100 =. The percent composition of sulfur in sulfuric acid (h2so4) is 32.7%. What is the percent composition of sulfur in h2so4? The percent composition of sulfur in sulfuric acid (h₂so₄).
Solved 21) Calculate the mass percent composition of sulfur
This answer has been confirmed as correct and. 32.7% explanation to determine the percent composition of sulfur in sulfuric acid. The molar mass of sulfur (s) is 32.06 g/mol, and the molar mass of h2so4 is 98.09 g/mol. The percent composition of sulfur in h2so4 is 32.7%. The percent composition of sulfur in h₂so₄ is:
H 2 S content and sulfur isotope composition of hydrogen sulfide and
The question is asking for the percent composition of sulfur in the chemical. Log in for more information. This is found by dividing the molar mass of sulfur by the total. The molar mass of sulfur (s) is 32.06 g/mol, and the molar mass of h2so4 is 98.09 g/mol. The percent composition of sulfur in sulfuric acid (h2so4) is 32.7%.
SOLVED Calculate the percent composition of H2SO4. Then calculate the
The percent composition of sulfur in sulfuric acid (h2so4) is 32.7%. 32.7% explanation to determine the percent composition of sulfur in sulfuric acid. The percent composition of sulfur in h2so4 is 32.7%. This answer has been confirmed as correct and. What is the percent composition of sulfur in h2so4?
[ANSWERED] What is the percent composition by mass of sulfur in NH4
The question is asking for the percent composition of sulfur in the chemical. 32.7% is the percent composition of sulfur in h2so4. The percent composition of sulfur in sulfuric acid (h2so4) is 32.7%. The percent composition of sulfur in sulfuric acid (h₂so₄) is approximately 32.65%, which aligns closely with option a. The percent composition of sulfur in h₂so₄ is:
This Answer Has Been Confirmed As Correct And.
32.7% explanation to determine the percent composition of sulfur in sulfuric acid. The percent composition of sulfur in h₂so₄ is: The molar mass of sulfur (s) is 32.06 g/mol, and the molar mass of h2so4 is 98.09 g/mol. The percent composition of sulfur in sulfuric acid (h₂so₄) is approximately 32.65%, which aligns closely with option a.
What Is The Percent Composition Of Sulfur In H2So4?
The question is asking for the percent composition of sulfur in the chemical. Log in for more information. This is found by dividing the molar mass of sulfur by the total. % composition = ( mass s / mass h2so4 ) × 100 = 32.08/ 98.10 × 100 =.
The Percent Composition Of Sulfur In Sulfuric Acid (H2So4) Is 32.7%.
The percent composition of sulfur in h2so4 is 32.7%. 32.7% is the percent composition of sulfur in h2so4.

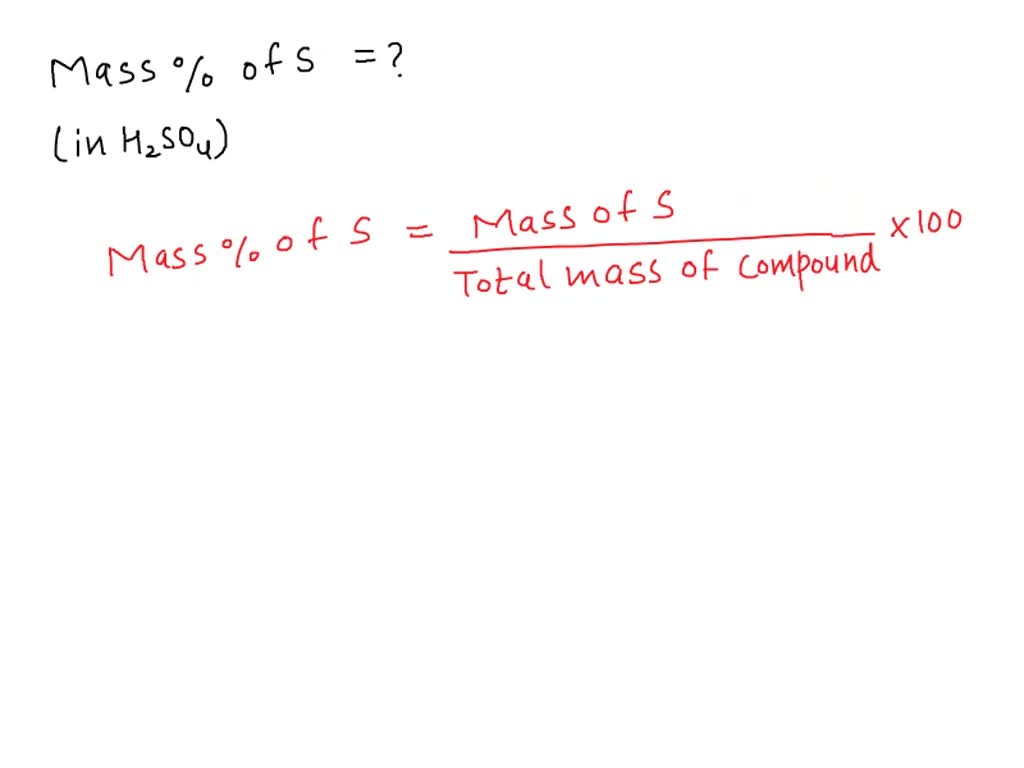





![[ANSWERED] What is the percent composition by mass of sulfur in NH4](https://media.kunduz.com/media/sug-question-candidate/20220421152511556337-3986252.jpg?h=512)