What Parts Of Dalton S Theory Are Incorrect
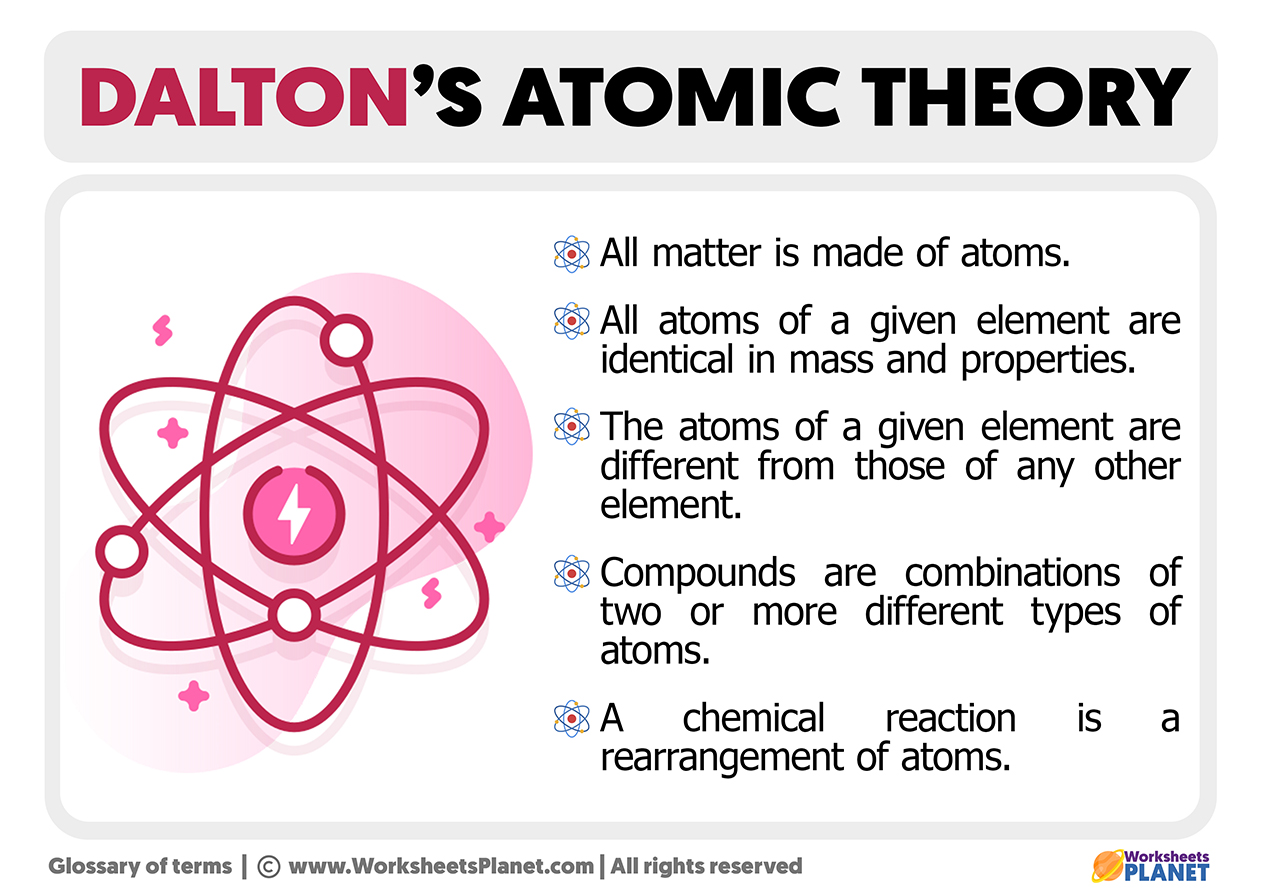
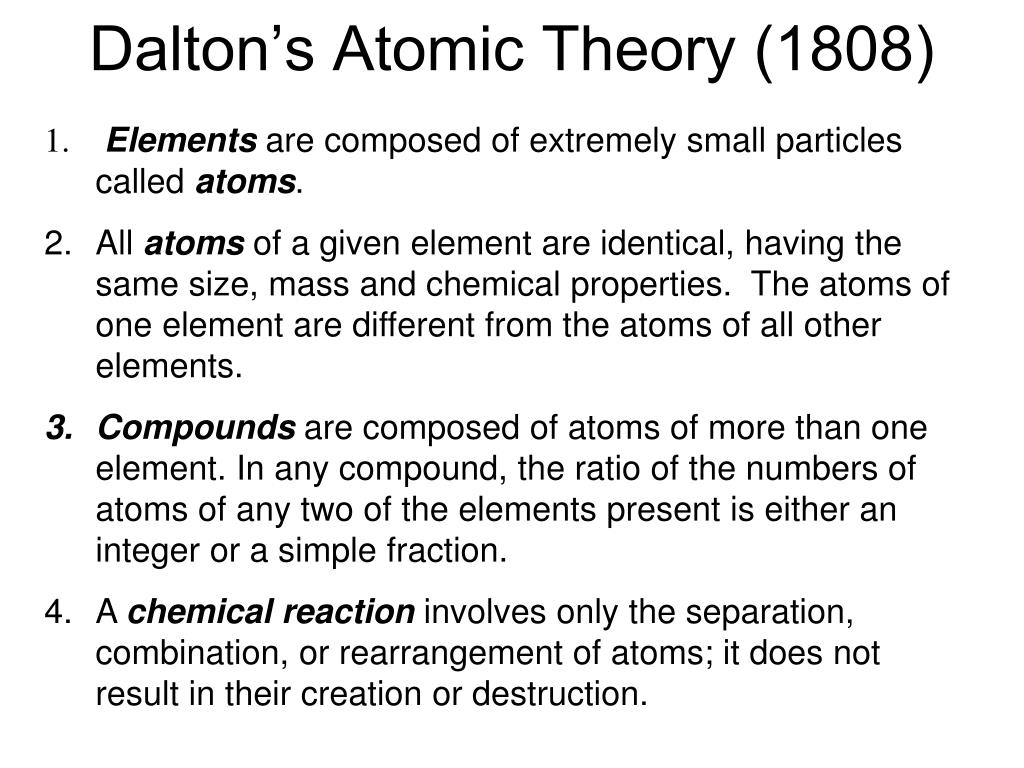
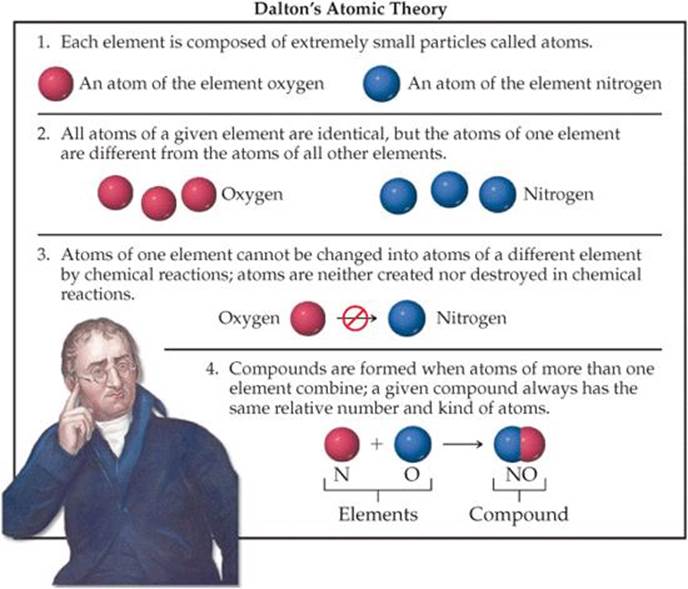
What Parts Of Dalton S Theory Are Incorrect - Atoms are never changed into atoms of another element. Dalton's atomic theory had several points that have been proven incorrect by modern science: This theory did not define subatomic particles: Look no further, as this article will delve into the parts of dalton’s theory that have been proven incorrect. (a) atoms of different elements differ in mass. According to dalton’s atomic theory, it stated that atoms were indivisible. The incorrect postulates of the dalton's atomic theory are : Dalton stated that atoms of a given. Chemical reactions occur when atoms are separated, joined, or rearranged. Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model.
According to dalton’s atomic theory, it stated that atoms were indivisible. This theory did not define subatomic particles: Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model. John dalton's atomic theory has been modified over time due to advancements in scientific knowledge. Dalton's atomic theory had several points that have been proven incorrect by modern science: Atoms are never changed into atoms of another element. (a) atoms of different elements differ in mass. Dalton stated that atoms of a given. The incorrect postulates of the dalton's atomic theory are : Chemical reactions occur when atoms are separated, joined, or rearranged.
Look no further, as this article will delve into the parts of dalton’s theory that have been proven incorrect. (a) atoms of different elements differ in mass. Dalton stated that atoms of a given. Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model. John dalton's atomic theory has been modified over time due to advancements in scientific knowledge. Atoms are never changed into atoms of another element. The incorrect postulates of the dalton's atomic theory are : Chemical reactions occur when atoms are separated, joined, or rearranged. This theory did not define subatomic particles: According to dalton’s atomic theory, it stated that atoms were indivisible.
Dalton's Atomic Theory Postulate, Limitations [Teachoo]
Atoms are never changed into atoms of another element. Dalton stated that atoms of a given. Chemical reactions occur when atoms are separated, joined, or rearranged. According to dalton’s atomic theory, it stated that atoms were indivisible. (a) atoms of different elements differ in mass.
John daltons atomic theory constructiondolf
Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model. John dalton's atomic theory has been modified over time due to advancements in scientific knowledge. Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms are never changed into atoms of another element. Dalton's atomic theory had several.
Dalton Atomic Theory
(a) atoms of different elements differ in mass. Dalton's atomic theory had several points that have been proven incorrect by modern science: Atoms are never changed into atoms of another element. John dalton's atomic theory has been modified over time due to advancements in scientific knowledge. The incorrect postulates of the dalton's atomic theory are :
PPT Dalton’s Atomic Theory (1808) PowerPoint Presentation, free
Dalton's atomic theory had several points that have been proven incorrect by modern science: Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model. Chemical reactions occur when atoms are separated, joined, or rearranged. Look no further, as this article will delve into the parts of dalton’s theory that.
John Dalton Experiment Atomic Theory
The incorrect postulates of the dalton's atomic theory are : Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model. John dalton's atomic theory has been modified over time due to advancements in scientific knowledge. Dalton stated that atoms of a given. Atoms are never changed into atoms of.
PPT John Dalton’s Atomic Theory 1803 PowerPoint Presentation, free
Dalton stated that atoms of a given. John dalton's atomic theory has been modified over time due to advancements in scientific knowledge. Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model. According to dalton’s atomic theory, it stated that atoms were indivisible. (a) atoms of different elements differ.
Dalton Atomic Theory
John dalton's atomic theory has been modified over time due to advancements in scientific knowledge. Dalton stated that atoms of a given. (a) atoms of different elements differ in mass. Chemical reactions occur when atoms are separated, joined, or rearranged. Dalton's atomic theory had several points that have been proven incorrect by modern science:
Understanding John Dalton's Atomic Theory of Matter Britannica
Look no further, as this article will delve into the parts of dalton’s theory that have been proven incorrect. Chemical reactions occur when atoms are separated, joined, or rearranged. Dalton stated that atoms of a given. This theory did not define subatomic particles: (a) atoms of different elements differ in mass.
Dalton's Atomic Theory Graphic Education
This theory did not define subatomic particles: Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model. Chemical reactions occur when atoms are separated, joined, or rearranged. The incorrect postulates of the dalton's atomic theory are : Atoms are never changed into atoms of another element.
Atomic theory dalton volfhawaii
According to dalton’s atomic theory, it stated that atoms were indivisible. The incorrect postulates of the dalton's atomic theory are : Dalton's atomic theory had several points that have been proven incorrect by modern science: Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model. Dalton stated that atoms.
Dalton Stated That Atoms Of A Given.
(a) atoms of different elements differ in mass. Atoms are never changed into atoms of another element. Look no further, as this article will delve into the parts of dalton’s theory that have been proven incorrect. The incorrect postulates of the dalton's atomic theory are :
This Theory Did Not Define Subatomic Particles:
Dalton's atomic theory had several points that have been proven incorrect by modern science: John dalton's atomic theory has been modified over time due to advancements in scientific knowledge. Study with quizlet and memorize flashcards containing terms like which part of dalton's theory was incorrect?, how is the bohr model. According to dalton’s atomic theory, it stated that atoms were indivisible.
![Dalton's Atomic Theory Postulate, Limitations [Teachoo]](https://d1avenlh0i1xmr.cloudfront.net/7157a4ce-b409-4ea0-a51b-c03a0ab9a986/dalton-atomic-theory-(ndivisible-atom)-teachoo-01.jpg)