What Species Has The Electron Configuration Ar 3D2
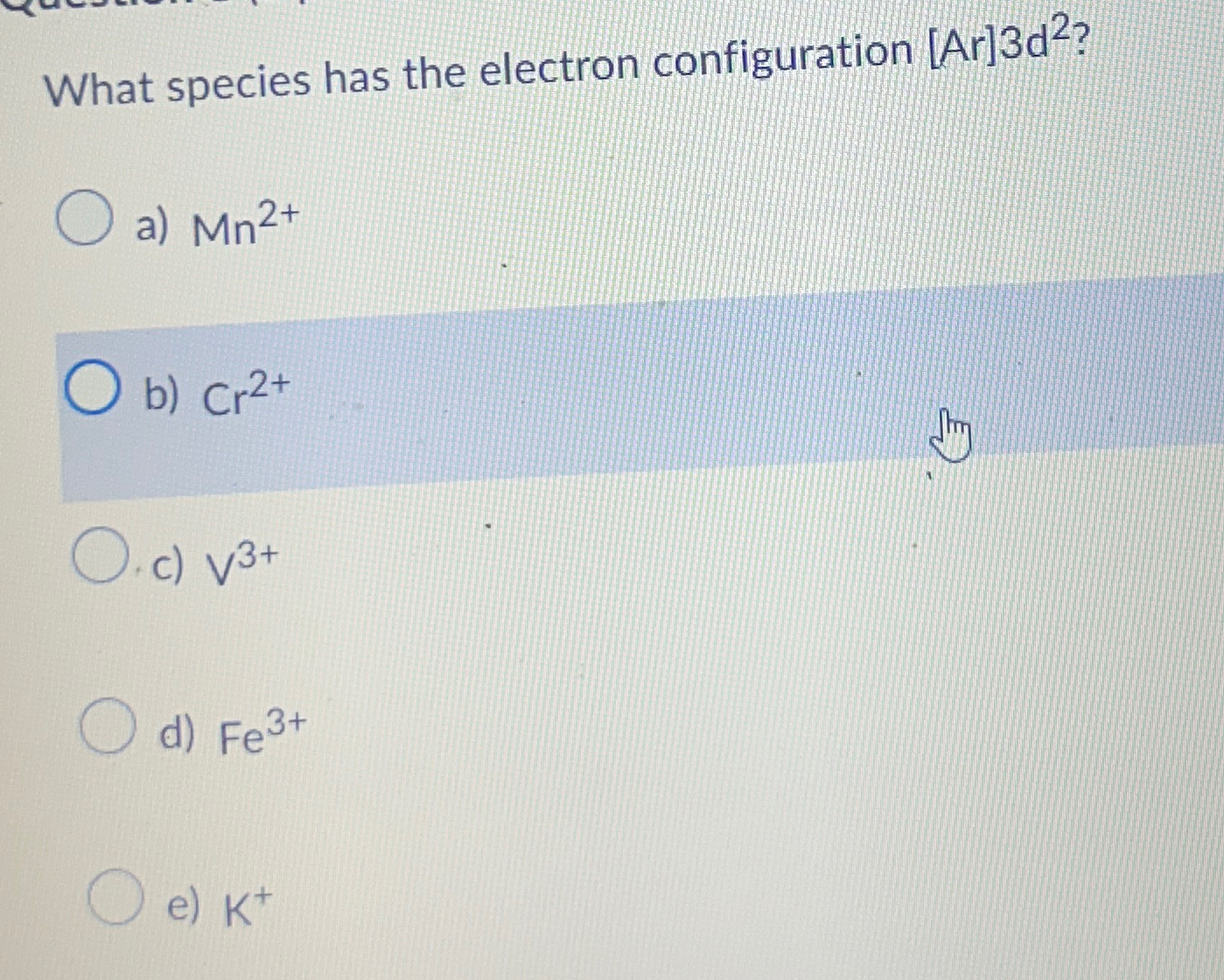
What Species Has The Electron Configuration Ar 3D2 - There are 2 steps to solve this one. What species has the electron configuration [ar]3d2? What is the electron configuration for the fe3+ ion? In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 Argon (a r) has an atomic number of 18. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. A chemical bond that involves. What species has the electron configuration [ar]3d2?
What species has the electron configuration [ar]3d2? Argon (a r) has an atomic number of 18. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. A chemical bond that involves. In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). There are 2 steps to solve this one. What is the electron configuration for the fe3+ ion? What species has the electron configuration [ar]3d2? The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10
What is the electron configuration for the fe3+ ion? What species has the electron configuration [ar]3d2? A chemical bond that involves. What species has the electron configuration [ar]3d2? The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. There are 2 steps to solve this one. Argon (a r) has an atomic number of 18. [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+.
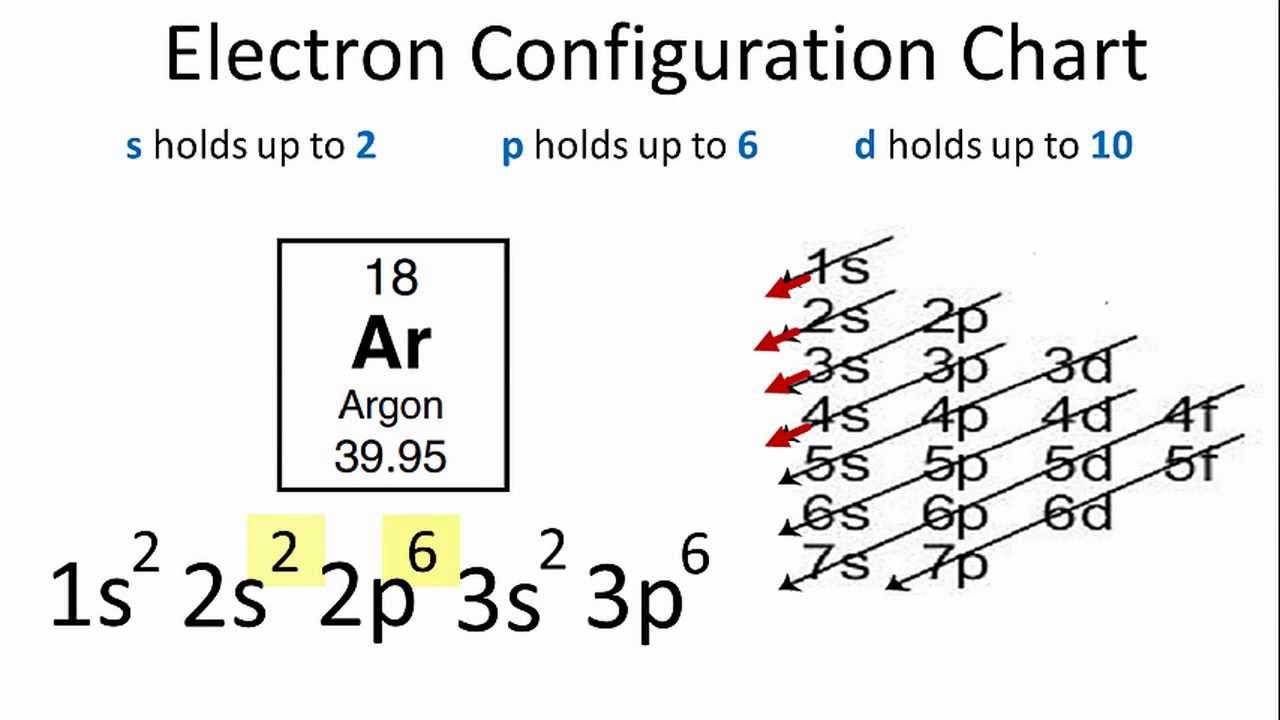
Argon Electron Configuration (Ar) with Orbital Diagram
A chemical bond that involves. There are 2 steps to solve this one. [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two.
Solved What species has the electron configuration
The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. Argon (a r) has an atomic number of 18. What species has the electron configuration [ar]3d2? What species has the electron configuration [ar]3d2? [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+.
Solved What species has the electron configuration
In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). What species has the electron configuration [ar]3d2? [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. What is.
Argon Electron Configuration (Ar) with Orbital Diagram
[ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. Argon (a r) has an atomic number of 18. [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 What species has the electron configuration [ar]3d2? There are 2 steps to solve this one.
What Species Has the Electron Configuration Ar 3d2
What species has the electron configuration [ar]3d2? What is the electron configuration for the fe3+ ion? Argon (a r) has an atomic number of 18. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. There are 2 steps to solve this one.
Answered What species has the electron… bartleby
[ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 A chemical bond that involves. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. What species has the electron configuration [ar]3d2? In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence).
What Species Has the Electron Configuration Ar 3d2
There are 2 steps to solve this one. The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. Argon (a r) has an atomic number of 18. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. What species has the electron configuration [ar]3d2?
What Species Has the Electron Configuration Ar 3d2
A chemical bond that involves. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. Argon (a r) has an atomic number of 18. What species has the electron configuration [ar]3d2?
What Species Has the Electron Configuration Ar 3d2
[ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). What is the electron configuration for the fe3+ ion? What species has the electron configuration [ar]3d2? A chemical bond that involves.
Electron Configuration of an Atom JavaLab
What species has the electron configuration [ar]3d2? [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. What is the electron configuration for the fe3+ ion? There are 2 steps to solve this one. [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10
The Electron Configuration [Ar]3D² Indicates An Atom That Has The Same Electron Configuration As Argon (Which Has 18 Electrons) Plus Two.
A chemical bond that involves. There are 2 steps to solve this one. What species has the electron configuration [ar]3d2? What species has the electron configuration [ar]3d2?
[Ne]3S23P6 What Species Has The Electron Configuration [Ar]3D2?A) Mn2+ B) Cr2+.
Argon (a r) has an atomic number of 18. [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). What is the electron configuration for the fe3+ ion?