What Type Of Ions Do Metals Form
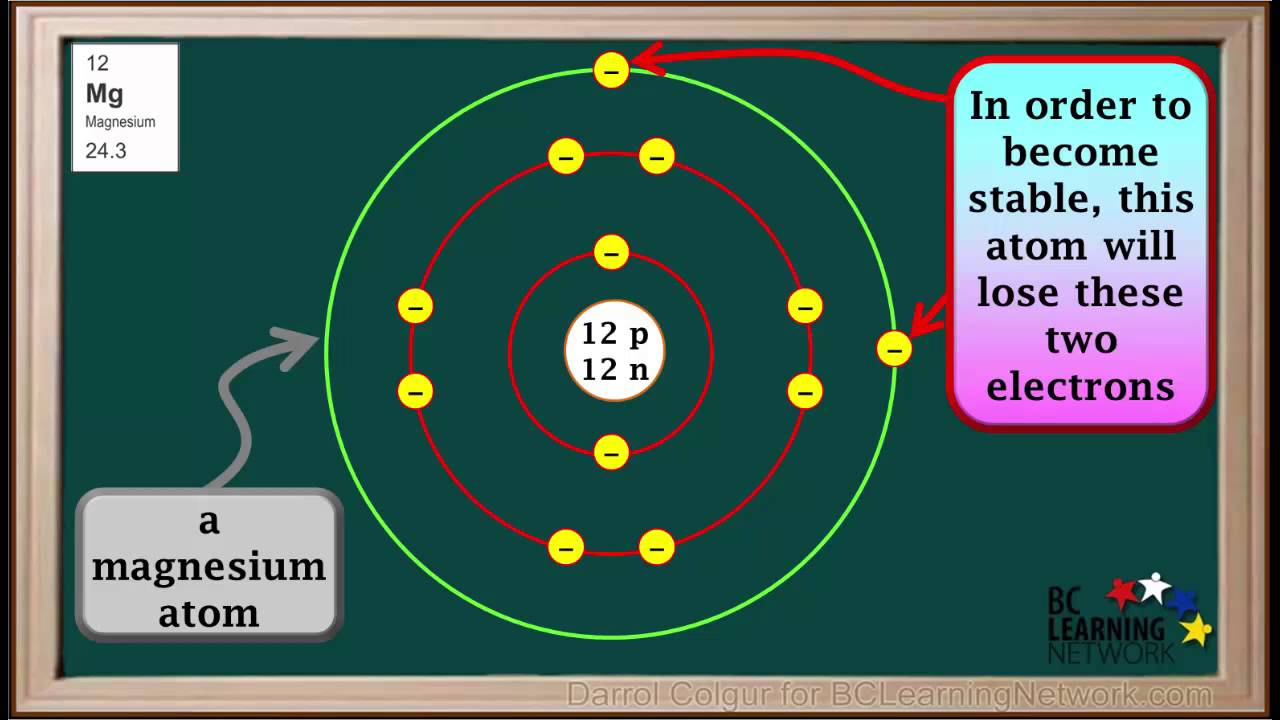
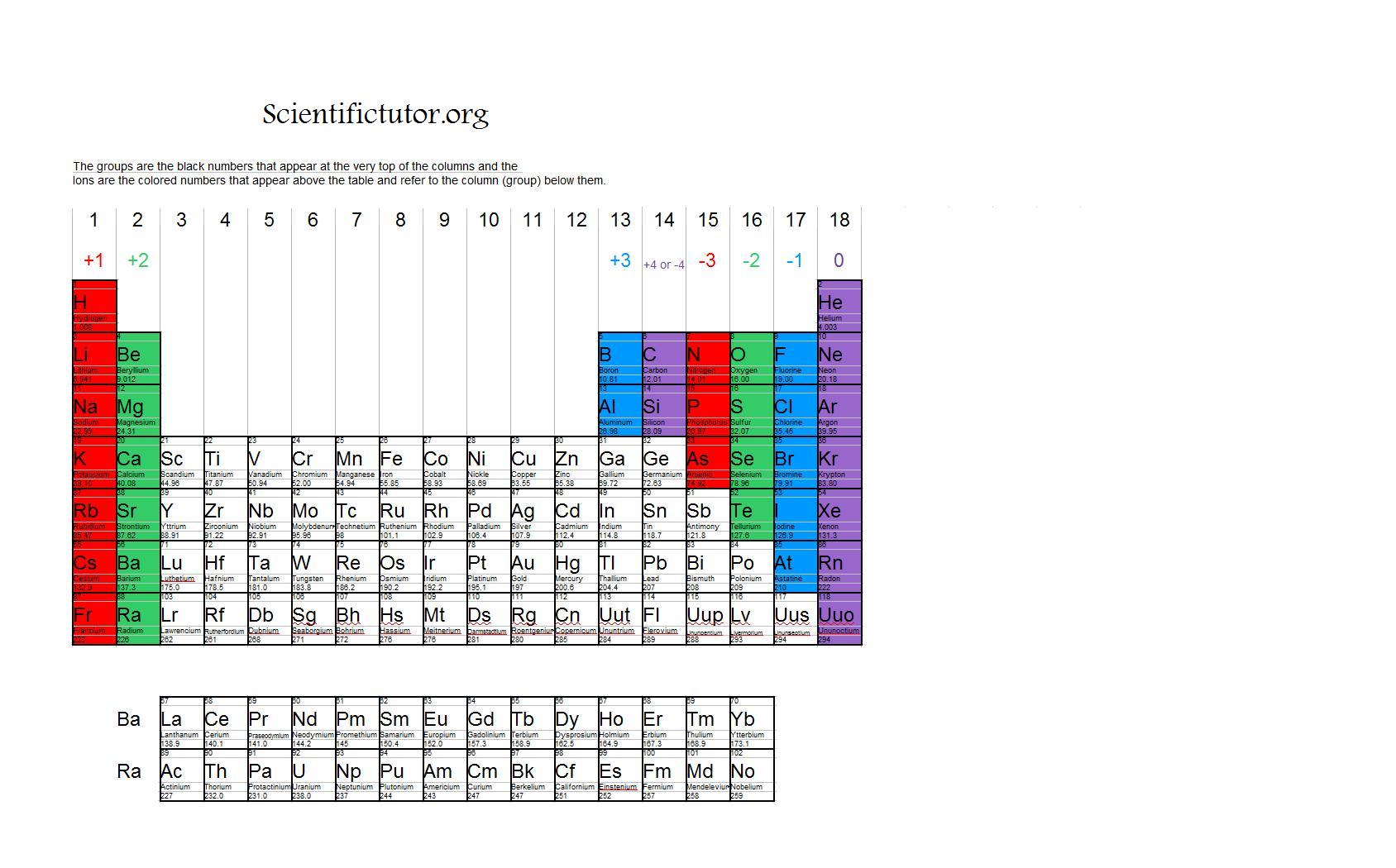
What Type Of Ions Do Metals Form - What type of ions do metals naturally form? Metal atoms lose electrons from their outer shell when they form ions: This occurs because metals tend to. Proton subatomic particle with a positive charge and a relative mass of 1. Metals typically form positively charged ions, known as cations, by losing electrons. An iron(ii) ion has a 2+ charge, and an. You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Alcl3, because aluminum loses three electrons and chlorine gains one electron.
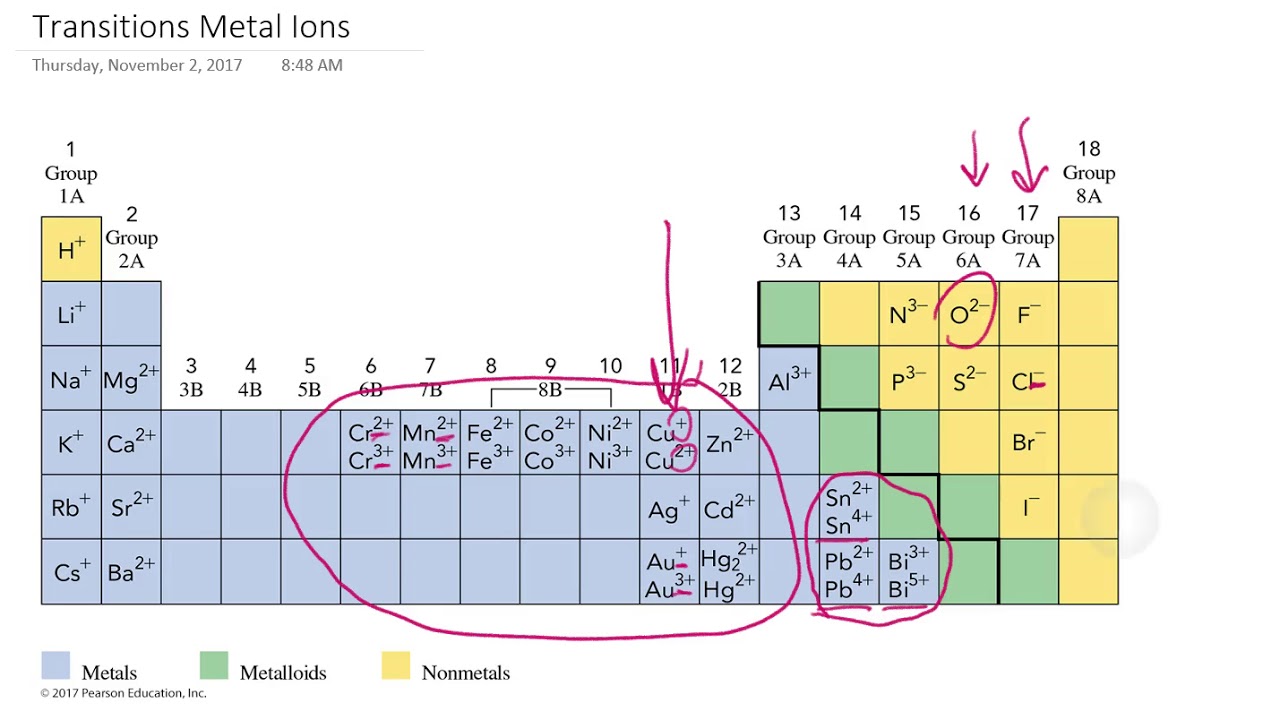
You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Proton subatomic particle with a positive charge and a relative mass of 1. Metals typically form positively charged ions, known as cations, by losing electrons. This occurs because metals tend to. Alcl3, because aluminum loses three electrons and chlorine gains one electron. An iron(ii) ion has a 2+ charge, and an. Metal atoms lose electrons from their outer shell when they form ions: What type of ions do metals naturally form?
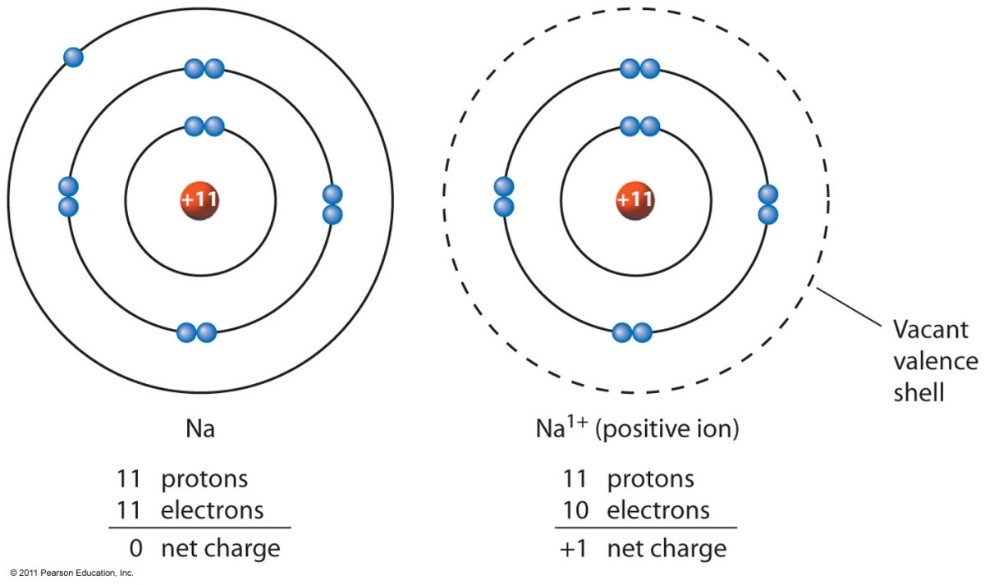
Metal atoms lose electrons from their outer shell when they form ions: You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. This occurs because metals tend to. What type of ions do metals naturally form? Proton subatomic particle with a positive charge and a relative mass of 1. An iron(ii) ion has a 2+ charge, and an. Metals typically form positively charged ions, known as cations, by losing electrons. Alcl3, because aluminum loses three electrons and chlorine gains one electron.
Do Metals Form Positive Or Negative Ions
Proton subatomic particle with a positive charge and a relative mass of 1. This occurs because metals tend to. You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Metals typically form positively charged ions, known as cations, by losing electrons. Metal atoms lose electrons from their outer shell when they form ions:
Do Metals Form Positive Or Negative Ions
Proton subatomic particle with a positive charge and a relative mass of 1. You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Metal atoms lose electrons from their outer shell when they form ions: An iron(ii) ion has a 2+ charge, and an. Alcl3, because aluminum loses three electrons and chlorine gains.
metals tend to form what kind of ions Lombardi Bothe1936
What type of ions do metals naturally form? Alcl3, because aluminum loses three electrons and chlorine gains one electron. An iron(ii) ion has a 2+ charge, and an. This occurs because metals tend to. Metals typically form positively charged ions, known as cations, by losing electrons.
Chem Ions Scientific Tutor
What type of ions do metals naturally form? Metal atoms lose electrons from their outer shell when they form ions: Alcl3, because aluminum loses three electrons and chlorine gains one electron. You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Proton subatomic particle with a positive charge and a relative mass of.
New AQA A2 Organic chemistry Transition metalsFormation of coloured
What type of ions do metals naturally form? You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. This occurs because metals tend to. Metal atoms lose electrons from their outer shell when they form ions: Proton subatomic particle with a positive charge and a relative mass of 1.
Do Metals Form Positive Or Negative Ions
This occurs because metals tend to. Metal atoms lose electrons from their outer shell when they form ions: Proton subatomic particle with a positive charge and a relative mass of 1. Metals typically form positively charged ions, known as cations, by losing electrons. What type of ions do metals naturally form?
Do Metals Form Positive Ions
Proton subatomic particle with a positive charge and a relative mass of 1. Alcl3, because aluminum loses three electrons and chlorine gains one electron. An iron(ii) ion has a 2+ charge, and an. This occurs because metals tend to. Metal atoms lose electrons from their outer shell when they form ions:
Do Metals Form Positive Or Negative Ions
You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. This occurs because metals tend to. Proton subatomic particle with a positive charge and a relative mass of 1. Metal atoms lose electrons from their outer shell when they form ions: An iron(ii) ion has a 2+ charge, and an.
Do Metals Form Positive Or Negative Ions
This occurs because metals tend to. An iron(ii) ion has a 2+ charge, and an. What type of ions do metals naturally form? You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Metal atoms lose electrons from their outer shell when they form ions:
Do Metals Form Positive Or Negative Ions Printable Form, Templates
Metals typically form positively charged ions, known as cations, by losing electrons. This occurs because metals tend to. Metal atoms lose electrons from their outer shell when they form ions: Proton subatomic particle with a positive charge and a relative mass of 1. You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges.
What Type Of Ions Do Metals Naturally Form?
You’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Proton subatomic particle with a positive charge and a relative mass of 1. Metals typically form positively charged ions, known as cations, by losing electrons. An iron(ii) ion has a 2+ charge, and an.
Alcl3, Because Aluminum Loses Three Electrons And Chlorine Gains One Electron.
Metal atoms lose electrons from their outer shell when they form ions: This occurs because metals tend to.