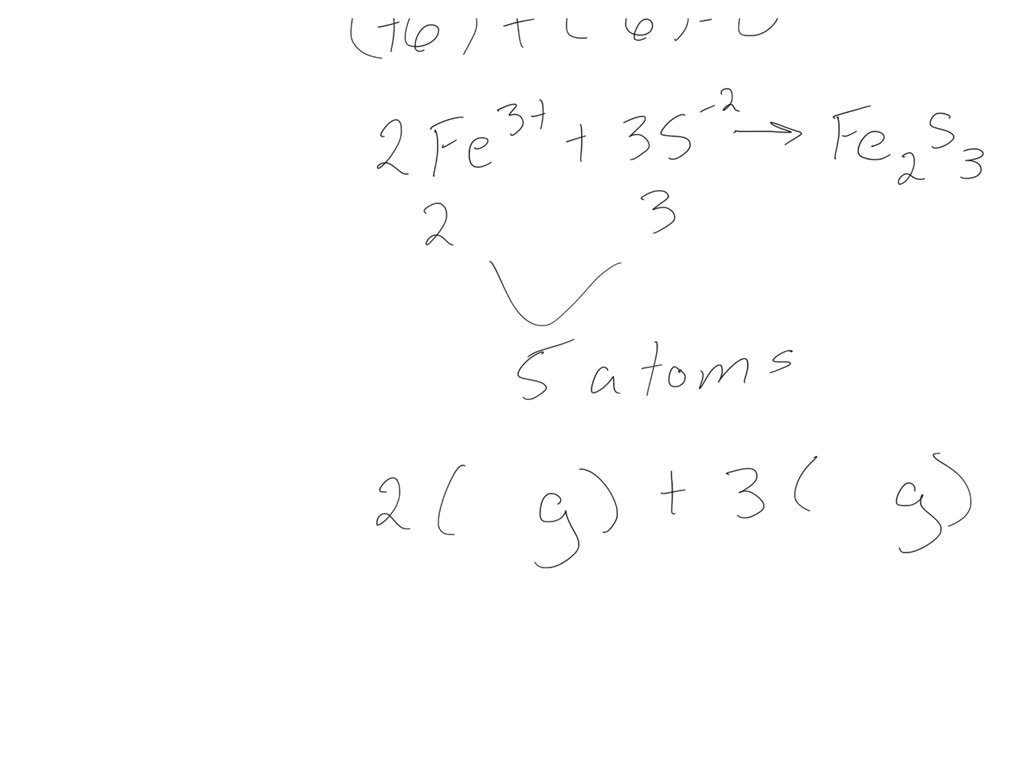What Is The Formula For The Compound Iron Iii Sulfate
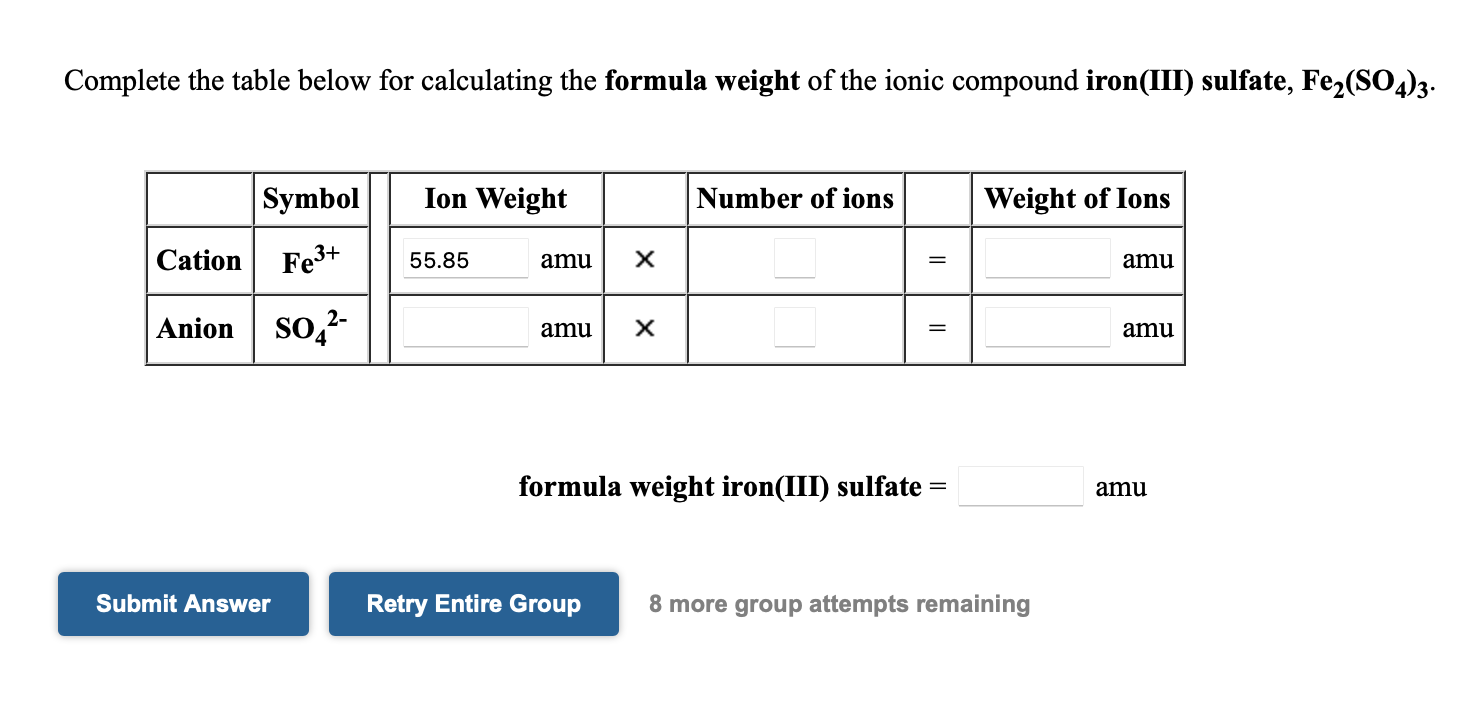
What Is The Formula For The Compound Iron Iii Sulfate - The iron iii sulfate formula is written as fe2(so4)3. So to neutralize both cation and anion, the total charge must be equal: To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. Additionally, the iron iii sulfate is composed of. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. Iron (iii) sulfate has a molar mass of 399.88 g/mol. 2 × ( + 3.
Additionally, the iron iii sulfate is composed of. Iron (iii) sulfate has a molar mass of 399.88 g/mol. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. The iron iii sulfate formula is written as fe2(so4)3. 2 × ( + 3. So to neutralize both cation and anion, the total charge must be equal: As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge.
So to neutralize both cation and anion, the total charge must be equal: 2 × ( + 3. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. The iron iii sulfate formula is written as fe2(so4)3. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. Iron (iii) sulfate has a molar mass of 399.88 g/mol. Additionally, the iron iii sulfate is composed of.
Solved Complete the table below for calculating the formula
The iron iii sulfate formula is written as fe2(so4)3. 2 × ( + 3. Iron (iii) sulfate has a molar mass of 399.88 g/mol. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. So to neutralize both cation and anion, the total charge must be equal:
Solved Complete the following for the compound iron(III)
Iron (iii) sulfate has a molar mass of 399.88 g/mol. 2 × ( + 3. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. The iron iii sulfate formula is written as fe2(so4)3. Additionally, the iron iii sulfate is composed of.
Ammonium iron(II) sulfate Alchetron, the free social encyclopedia
Additionally, the iron iii sulfate is composed of. Iron (iii) sulfate has a molar mass of 399.88 g/mol. 2 × ( + 3. So to neutralize both cation and anion, the total charge must be equal: The iron iii sulfate formula is written as fe2(so4)3.
Solved The compound iron(III) sulfate, Fe2(SO4)3 is soluble
Additionally, the iron iii sulfate is composed of. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. 2 × ( + 3. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. The iron iii sulfate formula is written.
Solved Use the Compounds in Aqueous Solution do to access
The iron iii sulfate formula is written as fe2(so4)3. 2 × ( + 3. So to neutralize both cation and anion, the total charge must be equal: Iron (iii) sulfate has a molar mass of 399.88 g/mol. Additionally, the iron iii sulfate is composed of.
Ammonium iron(II) sulfate Alchetron, the free social encyclopedia
2 × ( + 3. The iron iii sulfate formula is written as fe2(so4)3. Iron (iii) sulfate has a molar mass of 399.88 g/mol. So to neutralize both cation and anion, the total charge must be equal: As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge.
Sodium Thiosulfate for Hatcheries and Aquaculture Facilities Syndel
To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. So to neutralize both cation and anion, the total charge must be equal: Additionally, the iron iii sulfate is composed of. 2 × ( + 3. The iron iii sulfate formula is written as fe2(so4)3.
Examples of Iron Compounds Iron IronOxide IronSulfate
The iron iii sulfate formula is written as fe2(so4)3. 2 × ( + 3. Iron (iii) sulfate has a molar mass of 399.88 g/mol. So to neutralize both cation and anion, the total charge must be equal: Additionally, the iron iii sulfate is composed of.
SOLVED For the compound iron (III) sulfide, answer the following
Additionally, the iron iii sulfate is composed of. 2 × ( + 3. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. So to neutralize both cation and anion,.
Solved Use the Compounds in Aqueous Solution do to access
The iron iii sulfate formula is written as fe2(so4)3. So to neutralize both cation and anion, the total charge must be equal: To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. Additionally, the iron iii sulfate is composed of. As iron has + 3 oxidation state.
So To Neutralize Both Cation And Anion, The Total Charge Must Be Equal:
Iron (iii) sulfate has a molar mass of 399.88 g/mol. 2 × ( + 3. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. The iron iii sulfate formula is written as fe2(so4)3.
Additionally, The Iron Iii Sulfate Is Composed Of.
As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge.